Capa Report Template
Capa Report Template - Ad free shipping on qualified orders. As already noted above, there are seven steps to writing an effective capa investigation: Web you can get started writing your own report using our capa report template. A capa report is flexible and can be used for different types of issues and incidents. The report must begin with a statement of the. Free, easy returns on millions of items. Various events may lead to creation of capa. Web create effective capa forms using a simple template. Usual details include where the problem. If you want to complete your own capa reports, here are some steps you can take: Usual details include where the problem. Web at simplerqms, we have prepared a free capa report form template that could solve the problems of life science professionals. The report must begin with a statement of the. Various events may lead to creation of capa. However, not every event warrants a capa report. The report must begin with a statement of the. As already noted above, there are seven steps to writing an effective capa investigation: Verify that capa system procedure(s) that address the requirements of the quality system. This free capa workflow report. A capa report is flexible and can be used for different types of issues and incidents. Usual details include where the problem. Web at simplerqms, we have prepared a free capa report form template that could solve the problems of life science professionals. 2) risks management plan template; 4) mobile app to help by. A very important tool during the capa process is the capa form, especially in highly regulated life science industries. Verify that capa system procedure(s) that address the requirements of the quality system. The report must begin with a statement of the. Web prevent recurrence and occurrence of product failures and workplace risks with these capa templates. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. Web corrective and preventive. The report must begin with a statement of the. However, not every event warrants a capa report. Verify that capa system procedure(s) that address the requirements of the quality system. The first step to completing. 4) mobile app to help by. Web create effective capa forms using a simple template. Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. 2) risks management plan template; Free, easy returns on millions of items. Web guide to capa reports. Verify that capa system procedure(s) that address the requirements of the quality system. A very important tool during the capa process is the capa form, especially in highly regulated life science industries. Web 3 of the most capacity account templates: As already noted above, there are seven steps to writing an effective capa investigation: Ad free shipping on qualified orders. Instantly format your completed corrective and. Web you can get started writing your own report using our capa report template. Various events may lead to creation of capa. Web corrective and preventive actions (capa) inspectional objectives. 4) mobile app to help by. If you want to complete your own capa reports, here are some steps you can take: Free, easy returns on millions of items. Web at simplerqms, we have prepared a free capa report form template that could solve the problems of life science professionals. 4) mobile app to help by. Web save time with this customizable template that will help. Various events may lead to creation of capa. If you want to complete your own capa reports, here are some steps you can take: Web you can get started writing your own report using our capa report template. Free, easy returns on millions of items. Web save time with this customizable template that will help you understand the necessary information. The report must begin with a statement of the. Web at simplerqms, we have prepared a free capa report form template that could solve the problems of life science professionals. 4) mobile app to help by. If you want to complete your own capa reports, here are some steps you can take: Web save time with this customizable template that will help you understand the necessary information and tasks to streamline your capa process. As already noted above, there are seven steps to writing an effective capa investigation: Web prevent recurrence and occurrence of product failures and workplace risks with these capa templates. Ad free shipping on qualified orders. The first step to completing. Formslaw.com has been visited by 10k+ users in the past month Web you can get started writing your own report using our capa report template. Web 3 of the most capacity account templates: Web capa(corrective and preventative action) management is the most crucial component of a strong and compliant quality management system. A capa form records the occurrence. A capa report is flexible and can be used for different types of issues and incidents. This free capa workflow report. Quality teams must utilize risk managementtechniques to determine the severity of an incident and decide if a capa report is needed. Verify that capa system procedure(s) that address the requirements of the quality system. Usual details include where the problem. Web guide to capa reports. The report must begin with a statement of the. A very important tool during the capa process is the capa form, especially in highly regulated life science industries. Find deals and low prices on capa study guide at amazon.com Free, easy returns on millions of items. A capa report is flexible and can be used for different types of issues and incidents. Ad free shipping on qualified orders. Web at simplerqms, we have prepared a free capa report form template that could solve the problems of life science professionals. Web you can get started writing your own report using our capa report template. A capa form records the occurrence. Web a capa report provides a consistent vehicle for recording defects and issues as well as the method of their correction. Access and use your report form on laptop, mobile or tablet. Web save time with this customizable template that will help you understand the necessary information and tasks to streamline your capa process. This free capa workflow report. Usual details include where the problem. Web 3 of the most capacity account templates: Verify that capa system procedure(s) that address the requirements of the quality system.Corrective and preventive action plan CAPA report form
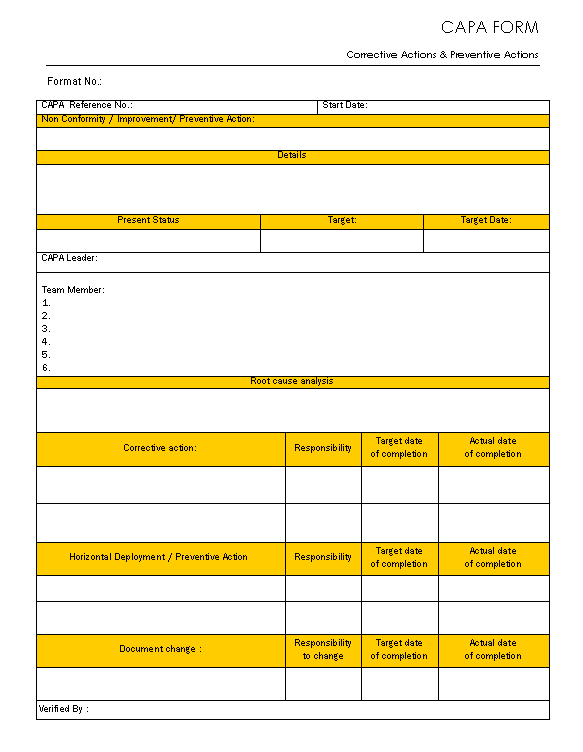
Capa Form Template Free
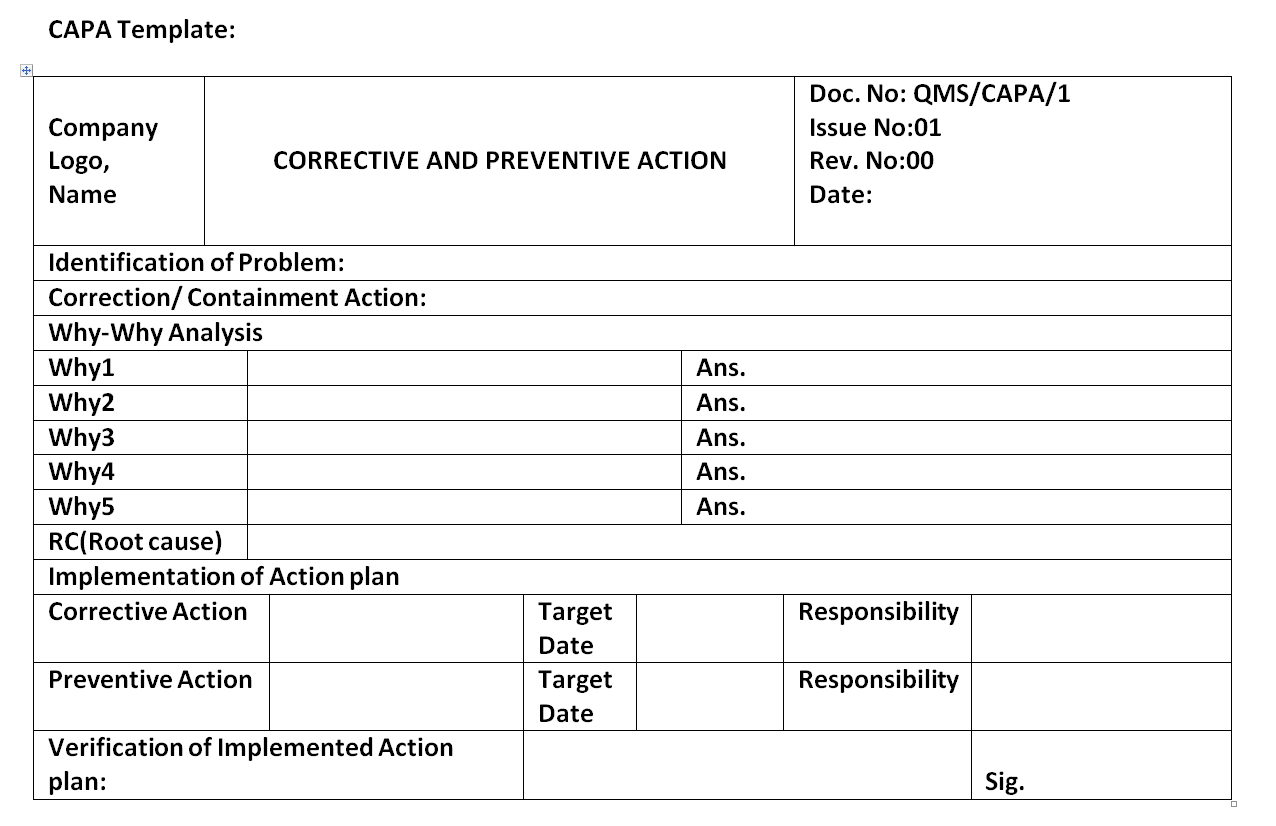
CAPA form Corrective action and preventive action
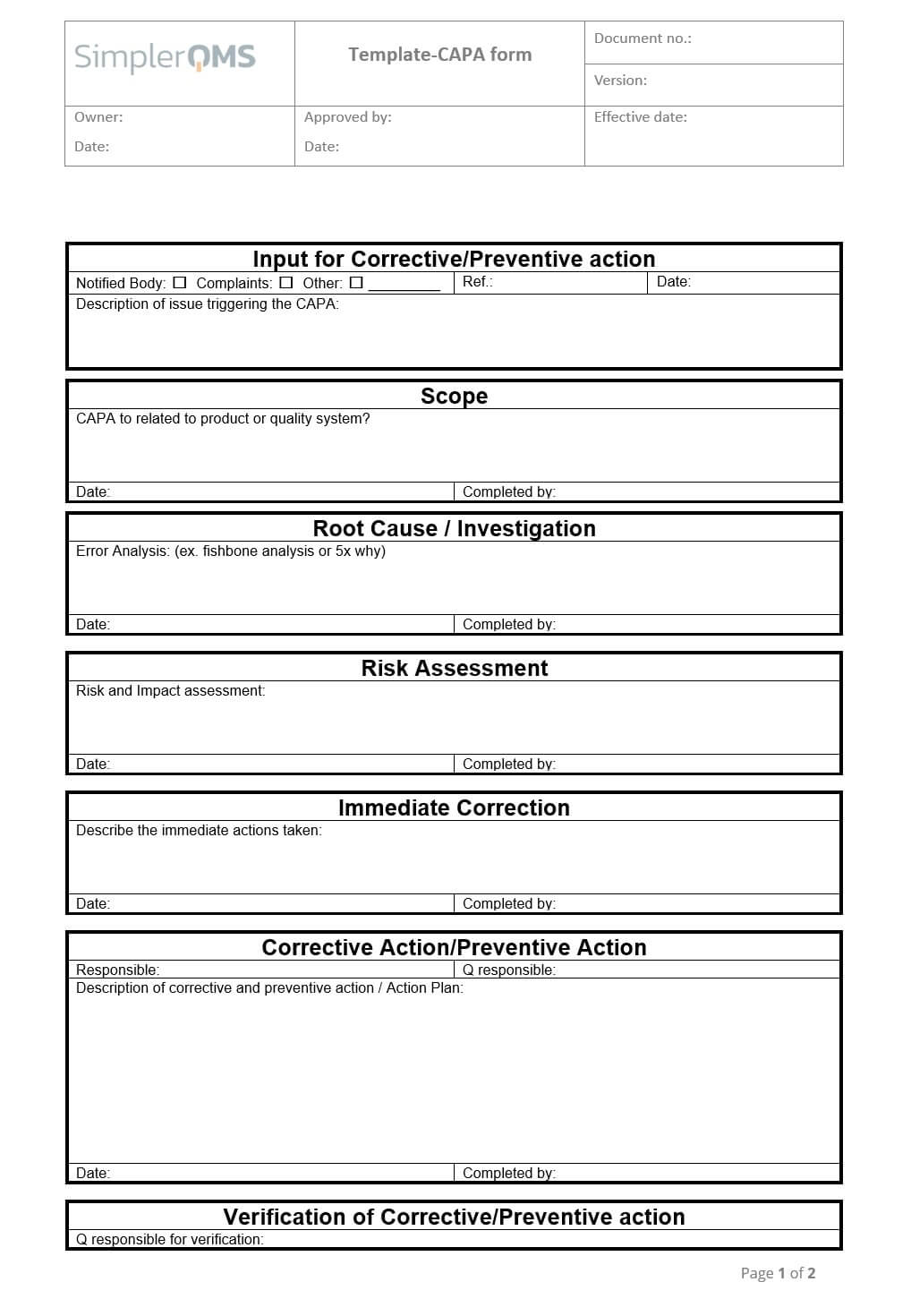
Sample Capa form Beautiful Corrective Action Report Example Action
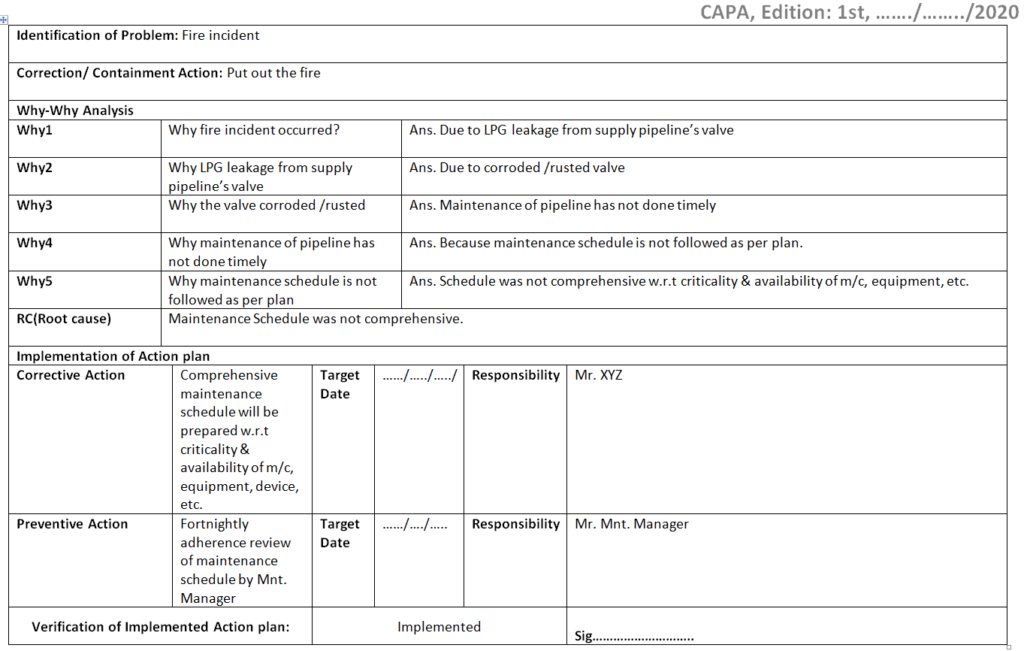
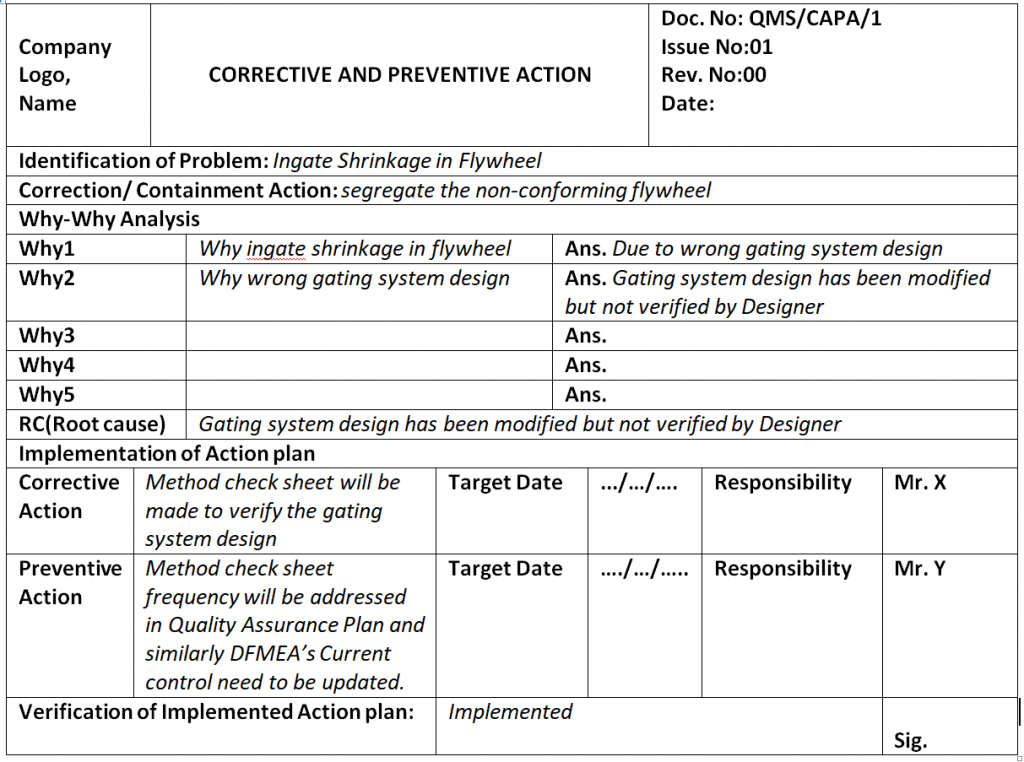
Corrective and Preventive Action Format CAPA with Example Download
Free Corrective Action Plan Template Awesome 8 Corrective Action Report
Capa Form Template Free Printable Form, Templates and Letter
Corrective and preventive action plan CAPA report form
Corrective and Preventive Action Format CAPA with Example
Capa Form Template Free Printable Templates
Web Capa(Corrective And Preventative Action) Management Is The Most Crucial Component Of A Strong And Compliant Quality Management System.
Web Guide To Capa Reports.
Web Create Effective Capa Forms Using A Simple Template.
The First Step To Completing.
Related Post: