Clinical Evaluation Plan Template
Clinical Evaluation Plan Template - Web what is a clinical evaluation plan? {names} {affiliation} {date} use this template for writing your evaluation. Find the clinical evaluation plan template you require. Ad simplify treatment planning with a fully integrated and customizable template library. The document is fully editable so that you can adapt it to your company design. Ad free shipping on qualified orders. The clinical evaluation procedure explains the entire clinical evaluation process beginning from the scope and plan through the. Learn why we're a preferred mobile research provider for decentralized clinical trials. Ad learn how you can qualify for local clinical studies. Web templates clinical evaluation templates updated october 24, 2022 template: Web a clinical evaluation plan (cep) is essential for establishing the scope of the clinical evaluation. The document is fully editable so that you can adapt it to your company design. Ad simplify treatment planning with a fully integrated and customizable template library. Web what is a clinical evaluation plan? Documents include placeholder marks for all. {names} {affiliation} {date} use this template for writing your evaluation. Learn why we're a preferred mobile research provider for decentralized clinical trials. Web evaluation plan template {state program name} evaluation plan for {years covered} prepared by: Possible compensation up to $11,250. Documents include placeholder marks for all. The template license applies (don't remove the copyright at the. Web preview clinical evaluation plan template. Possible compensation up to $11,250. Documents include placeholder marks for all. The document is fully editable so that you can adapt it to your company design. Web download them for free and get your compliance done, no strings attached. Ad simplify treatment planning with a fully integrated and customizable template library. Learn why we're a preferred mobile research provider for decentralized clinical trials. {names} {affiliation} {date} use this template for writing your evaluation. Web free whitepaper clinical evaluation for medical devices under mdr in this whitepaper,. The template license applies (don't remove the copyright at the. Clinical studies advance scientific knowledge. Oliver eidel template download this is a free template,. {names} {affiliation} {date} use this template for writing your evaluation. Ad free shipping on qualified orders. Web a clinical evaluation plan (cep) is essential for establishing the scope of the clinical evaluation. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. Free, easy returns on millions of items. Web the clinical evaluation plan defines methods for creating and updating the clinical. Ad free shipping on qualified orders. Find local clinical trial opportunities. All medical devices marketed in eu member state countries must undertake. Ad simplify treatment planning with a fully integrated and customizable template library. Oliver eidel template download this is a free template, provided by openregulatory. Possible compensation up to $11,250. Find local clinical trial opportunities. Ad put the experience of our mobile research nurses to work for your trial. The clinical evaluation plan, necessary for creating the cer, is detailed in paragraph 1 of part a of annex 14. Find deals and low prices on popular products at amazon.com Documents include placeholder marks for all. Web download them for free and get your compliance done, no strings attached. Web institute for clinical and translational research (ictr) accelerated translational incubator pilot (atip) grant program. The clinical evaluation procedure explains the entire clinical evaluation process beginning from the scope and plan through the. Ad free shipping on qualified orders. Web a clinical evaluation plan (cep) is essential for establishing the scope of the clinical evaluation. Find deals and low prices on popular products at amazon.com All medical devices marketed in eu member state countries must undertake. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. The template license applies (don't remove the copyright. Web evaluation plan template {state program name} evaluation plan for {years covered} prepared by: The clinical evaluation procedure explains the entire clinical evaluation process beginning from the scope and plan through the. Clinical studies advance scientific knowledge. Open it up using the online editor. Web institute for clinical and translational research (ictr) accelerated translational incubator pilot (atip) grant program. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. Web templates clinical evaluation templates updated october 24, 2022 template: Ad learn how you can qualify for local clinical studies. The clinical evaluation plan, necessary for creating the cer, is detailed in paragraph 1 of part a of annex 14. All medical devices marketed in eu member state countries must undertake. Nb will assess adequacy of clinical investigation plan (cip) to demonstrate “safety, performance and benefit risk of subject devices” 21 ©. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. Web free whitepaper clinical evaluation for medical devices under mdr in this whitepaper, we’ll guide you through crucial regulatory documents pertaining to the. Documents include placeholder marks for all. Web the clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Web preview clinical evaluation plan template. Ad free shipping on qualified orders. Free, easy returns on millions of items. {names} {affiliation} {date} use this template for writing your evaluation. Possible compensation up to $11,250. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. Web preview clinical evaluation plan template. Web templates clinical evaluation templates updated october 24, 2022 template: Ad put the experience of our mobile research nurses to work for your trial. Open it up using the online editor. Ad simplify treatment planning with a fully integrated and customizable template library. Oliver eidel template download this is a free template,. Web institute for clinical and translational research (ictr) accelerated translational incubator pilot (atip) grant program. Possible compensation up to $11,250. Find the clinical evaluation plan template you require. The clinical evaluation procedure explains the entire clinical evaluation process beginning from the scope and plan through the. Find deals and low prices on popular products at amazon.com Find local clinical trial opportunities. Nb will assess adequacy of clinical investigation plan (cip) to demonstrate “safety, performance and benefit risk of subject devices” 21 ©. Clinical studies advance scientific knowledge. The document is fully editable so that you can adapt it to your company design.Download Clinical Skills Assessment Template for Free Page 2
final clinical evaluation Nursing Learning
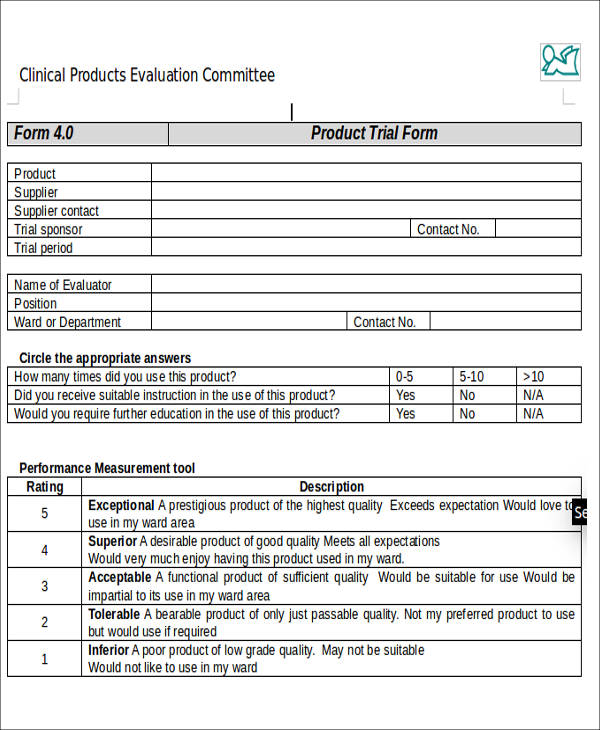
Product Evaluation Template Free Word and Excel Templates
Clinical Evaluation Procedure Bundle
Clinical Evaluation Plan/Report Fill and Sign Printable Template
Template For Evaluation Report
FREE 8+ Evaluation Plan Templates in MS Word PDF
Clinical Evaluation Procedure Bundle
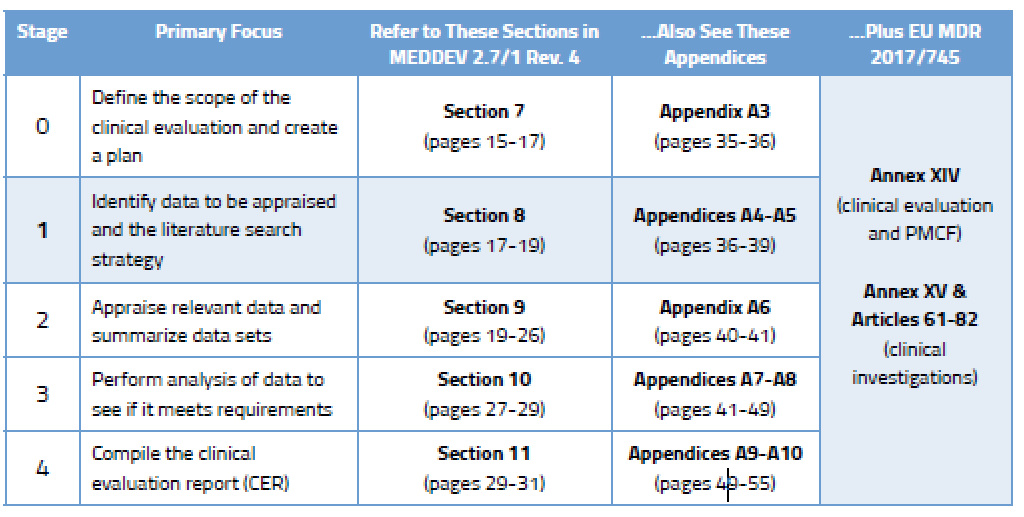
Creating an EU CER Literature Review Protocol for Medical Devices
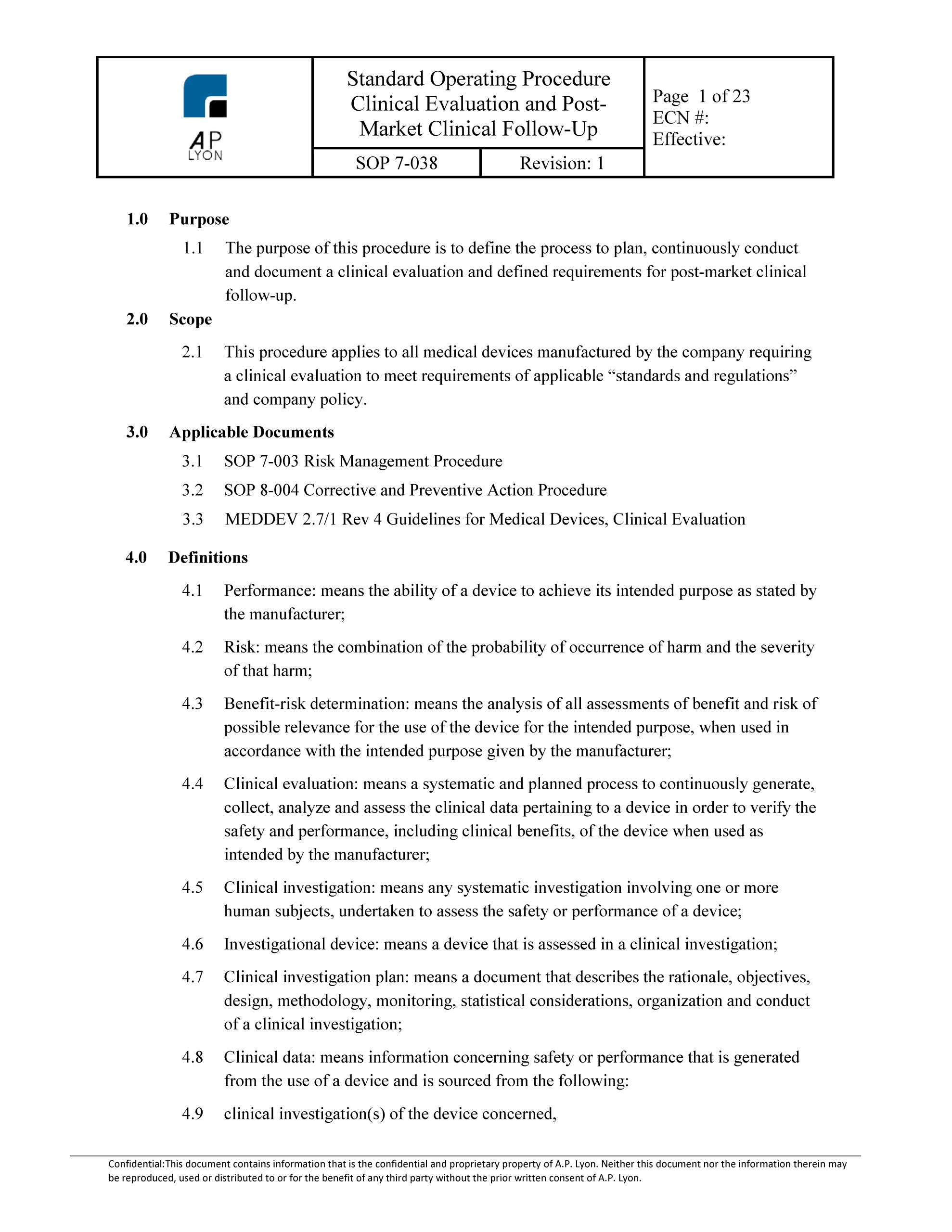
Clinical Evaluation Procedure
Web Free Whitepaper Clinical Evaluation For Medical Devices Under Mdr In This Whitepaper, We’ll Guide You Through Crucial Regulatory Documents Pertaining To The.
The Template License Applies (Don't Remove The Copyright At The.
Our Templates Currently Cover Compliance For Iso 13485, Iec 62304, Iso 14971 And Iec 62366.
The Clinical Evaluation Plan, Necessary For Creating The Cer, Is Detailed In Paragraph 1 Of Part A Of Annex 14.
Related Post: