Clinical Evaluation Report Template Mdr
Clinical Evaluation Report Template Mdr - Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. Web clinical evaluation is a systematic and planned continuous generation, collection, analysis and evaluation of clinical data. Guidance on pmcf plan template: Web updated october 24, 2022 template: A summary report of clinical investigation template is. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under the. Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. Web guidance on pmcf evaluation report template: Clinical evaluation assessment report (cear) template. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Web updated october 24, 2022 template: Oliver eidel template download this is a free template, provided by openregulatory. A summary report of clinical investigation template is. Clinical evaluation report sop & templates faq's what is. Web guidance on clinical evaluation (mdr) / performance evaluation (ivdr) of medical device software » in order to carry out the clinical data evaluation. Web clinical evaluation is a systematic and planned continuous generation, collection, analysis and evaluation of clinical data. It is primarily aimed at clinical evaluation reviewers, particularly. The. Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the clinical evaluation performed. The purpose of clinical evaluation. Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. It is primarily aimed at clinical evaluation reviewers, particularly.. It is primarily aimed at clinical evaluation reviewers, particularly. Clinical evaluation report sop & templates faq's what is. Guidance on pmcf plan template: Web this template applies to mdr annexes ix section 4 and annex x section 3. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical. Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Oliver eidel template download this is a free template, provided. Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. A summary report of clinical investigation template is. It is primarily aimed at clinical evaluation reviewers, particularly. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of. Web clinical evaluation is a systematic and planned continuous generation, collection, analysis and evaluation of clinical data. It also applies to assessments of technical documentations on a sampling basis for class iia/iib devices. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. Web before entering into the details of the requirements. A summary report of clinical investigation template is. Guidance on pmcf plan template: It is primarily aimed at clinical evaluation reviewers, particularly. Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under the. Web a clinical evaluation report sample & template for medical. Clinical evaluation report sop & templates faq's what is. The purpose of clinical evaluation. Web this template applies to mdr annexes ix section 4 and annex x section 3. The clinical evaluation report training course conference has. Web guidance on pmcf evaluation report template: Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. A summary report of clinical investigation template is. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. The purpose of clinical evaluation. Web an effective. It is primarily aimed at clinical evaluation reviewers, particularly. Guidance on pmcf plan template: Web guidance on clinical evaluation (mdr) / performance evaluation (ivdr) of medical device software » in order to carry out the clinical data evaluation. Web clinical evaluation is a systematic and planned continuous generation, collection, analysis and evaluation of clinical data. Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Web guidance on pmcf evaluation report template: The clinical evaluation report training course conference has. Web a clinical review report (cer) is a difficult technical document that summarily the process of clinical evaluation, a partial of all medical device regulatory submissions under the. Clinical evaluation assessment report (cear) template. Web this template applies to mdr annexes ix section 4 and annex x section 3. A summary report of clinical investigation template is. Web before entering into the details of the requirements associated to the clinical evaluation process, we remind that qualitymeddev offers a clinical evaluation report. Web clinical evaluation report is a document that has all necessary elements for conducting and reporting the clinical evaluation process required by mdr and additional. The purpose of clinical evaluation. Web the mdcg is working on a cip template and clinical investigation evaluation template, which are due in spring 2021. Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the clinical evaluation performed. Oliver eidel template download this is a free template, provided by openregulatory. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. Clinical evaluation report sop & templates faq's what is. Web guidance on pmcf evaluation report template: The clinical evaluation report training course conference has. Guidance on pmcf plan template: Oliver eidel template download this is a free template, provided by openregulatory. Web this template applies to mdr annexes ix section 4 and annex x section 3. Web before entering into the details of the requirements associated to the clinical evaluation process, we remind that qualitymeddev offers a clinical evaluation report. Web girish hirpara, regulatory consultant on kolabtree, provides a clinical evaluation report sample for medical devices to use as a template for mdr. Web a clinical evaluation report sample & template for medical device is a document that contains the conclusions of the clinical evaluation performed. Web guidance on clinical evaluation (mdr) / performance evaluation (ivdr) of medical device software » in order to carry out the clinical data evaluation. Web an effective clinical evaluation report template must enable a writer to draft an accurate summary of every individual piece of clinical evidence, as required by mdr. Web our writing team has developed easy to use and edit clinical evaluation report templates, procedures and sop's. Web the mdcg is working on a cip template and clinical investigation evaluation template, which are due in spring 2021. It is primarily aimed at clinical evaluation reviewers, particularly. Web a clinical rate report (cer) is a complex technical document that summaries an process of cellular review, ampere component of all medical device regulatory submissions. The purpose of clinical evaluation.Clinical Evaluation Report Template QualityMedDev
Post Market Clinical Follow Up (PMCF) Evaluation Report
Medical Device Report (MDR) Procedure
Clinical Evaluation Report Template Glendale Community regarding
Clinical Evaluation Report Template
Clinical Evaluation Procedure Bundle
Clinical Evaluation Procedure Bundle
Anatomage_Clinical_Evaluation_ReportTom_Navarro
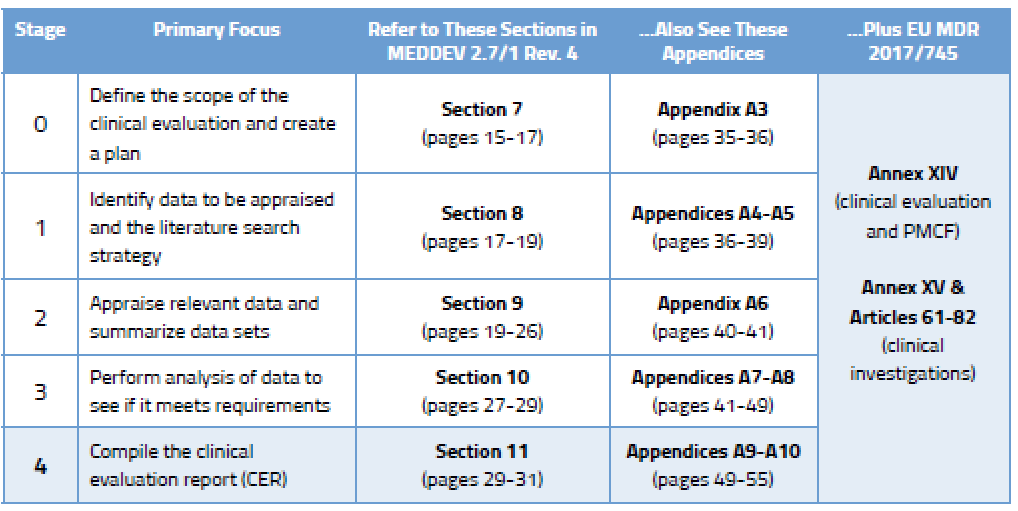
How To Write and Update Your EU CER Oriel STAT A MATRIX
Patient Report Form Template Download Best Template Ideas
Clinical Evaluation Assessment Report (Cear) Template.
A Summary Report Of Clinical Investigation Template Is.
It Also Applies To Assessments Of Technical Documentations On A Sampling Basis For Class Iia/Iib Devices.
Web Clinical Evaluation Report Is A Document That Has All Necessary Elements For Conducting And Reporting The Clinical Evaluation Process Required By Mdr And Additional.
Related Post: