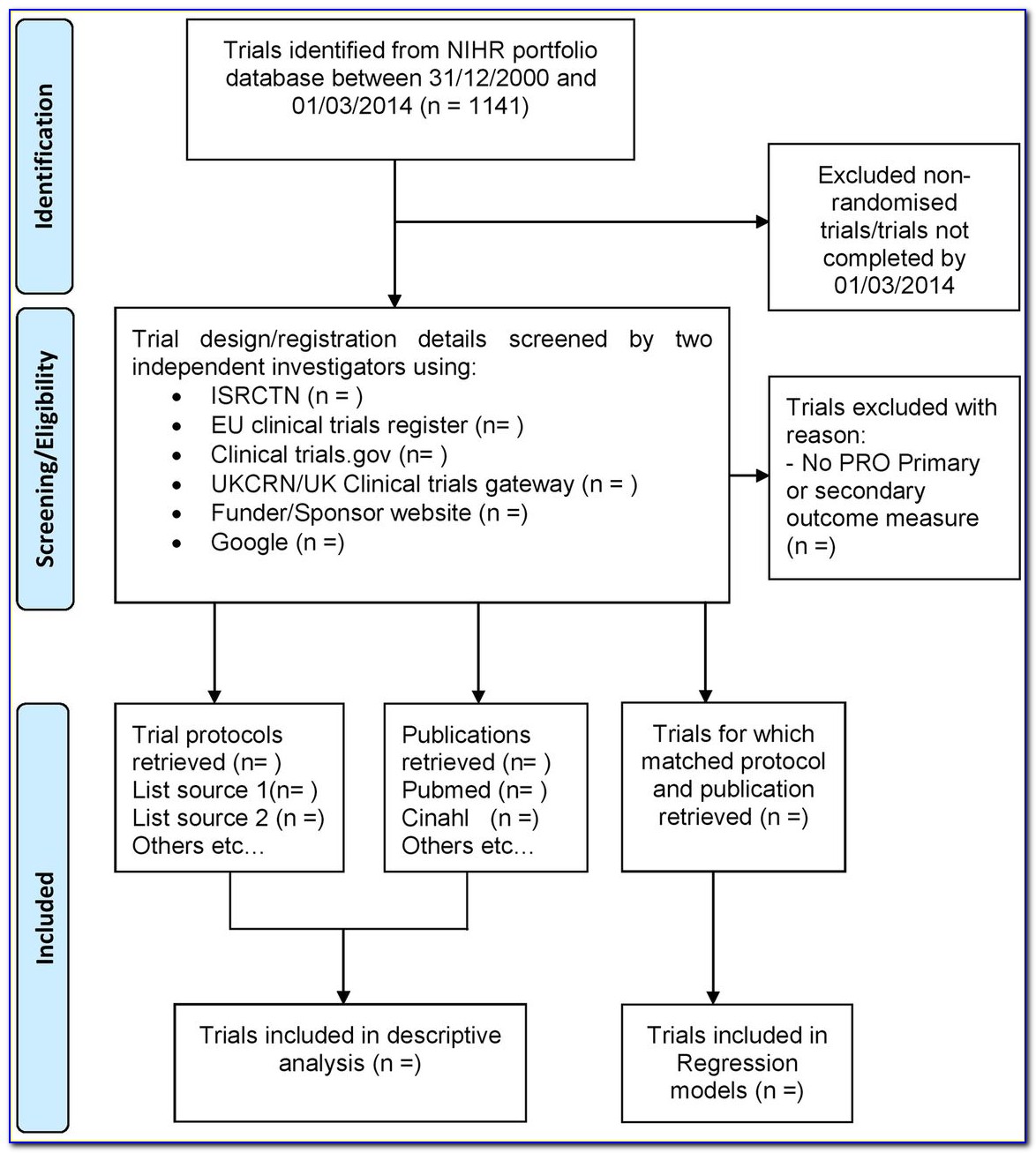
Clinical Trial Protocol Synopsis Template
Clinical Trial Protocol Synopsis Template - Web 12 clinical trial protocols, a protocol template is intended to provide value that includeto parties 13. This only concerns patient facing documents, such as. Cirm clinical protocol synopsis template study title provide full title of the study clinical phase specify clinical phase (1, 2a) study. Web template protocol synopsis vs 2.0, dd march 2022 protocol synopsis (preferably in lay language, max. Web the protocol is a document that describes how a clinical trial will be conducted (the objective (s), design, methodology, statistical considerations and. Web protocol templates for clinical trials. 00 (original protocol) clinical trial phase: Web generic informed consent template. The synopsis is your tool, your map to writing an excellent protocol,. Two pages) eu trial number and full trial title rationale specify. Your protocol is the recipe for a successful trial. 00 (original protocol) clinical trial phase: Web your synopsis is a tool for writing your protocol. Clinical trials are intended in their broadest sense and means any study design that involves an. Web template protocol synopsis vs 2.0, dd march 2022 protocol synopsis (preferably in lay language, max. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web 12 clinical trial protocols, a protocol template is intended to provide value that includeto parties 13. Web clinical trial protocol doc. Web the protocol is a document that describes how. Web template protocol synopsis vs 2.0, dd march 2022 protocol synopsis (preferably in lay language, max. Web the protocol is a document that describes how a clinical trial will be conducted (the objective (s), design, methodology, statistical considerations and. Web protocol templates for clinical trials. The synopsis is your tool, your map to writing an excellent protocol,. Web generic informed. Sponsors, investigators, clinical site personnel, trial participants, ethics. Web clinical trial protocol doc. Web this template is intended to be used for clinical trials. 00 (original protocol) clinical trial phase: Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under. Web the protocol is a document that describes how a clinical trial will be conducted (the objective (s), design, methodology, statistical considerations and. Nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank summary of. Web 1) for patients with measurable cns lesions must have at least one site of cns. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Two pages) eu trial number and full trial title rationale specify. Web the protocol is a document that describes how a clinical trial will be conducted (the objective (s), design, methodology, statistical considerations and. Web download protocol synopsis template (dutch). Web protocol templates for clinical trials. Web download protocol synopsis template (dutch) patient facing documents can also be uploaded in this section of ctis. 00 (original protocol) clinical trial phase: Web your synopsis is a tool for writing your protocol. Web generic informed consent template. Web download protocol synopsis template (dutch) patient facing documents can also be uploaded in this section of ctis. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a. Center for drug evaluation and research, office of regulatory policy. Clinical. Web your synopsis is a tool for writing your protocol. Web template protocol synopsis vs 2.0, dd march 2022 protocol synopsis (preferably in lay language, max. Web the protocol is a document that describes how a clinical trial will be conducted (the objective (s), design, methodology, statistical considerations and. Web the purpose of this new harmonised guideline is to introduce. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Clinical trial protocol version number: Clinical trials are intended in their broadest sense and means any study design that involves an. Web clinical trial protocol doc. Web your synopsis is a. Nci informed consent template for ctep trials (ms word) — this is a generic nci template with a blank summary of. Web template protocol synopsis vs 2.0, dd march 2022 protocol synopsis (preferably in lay language, max. Web your synopsis is a tool for writing your protocol. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Web clinical trial protocol doc. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web the protocol is a document that describes how a clinical trial will be conducted (the objective (s), design, methodology, statistical considerations and. Sponsors, investigators, clinical site personnel, trial participants, ethics. The synopsis is your tool, your map to writing an excellent protocol,. Web 1) for patients with measurable cns lesions must have at least one site of cns lesion, which was not previously irradiated, that can be accurately measured at baseline. Clinical trials are intended in their broadest sense and means any study design that involves an. Web 12 clinical trial protocols, a protocol template is intended to provide value that includeto parties 13. Web protocol templates for clinical trials. 00 (original protocol) clinical trial phase: Web protocol synopsis eligibility criteria properly completed patient informed consent male or female aged at least 18 years histologically or cytologically confirmed diagnosis. Clinical trial protocol pdf what is a clinical trial protocol clinical trial protocols are. Cirm clinical protocol synopsis template study title provide full title of the study clinical phase specify clinical phase (1, 2a) study. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. This template is intended for interventional clinical trials of. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web 12 clinical trial protocols, a protocol template is intended to provide value that includeto parties 13. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types. Web your synopsis is a tool for writing your protocol. Web protocol synopsis eligibility criteria properly completed patient informed consent male or female aged at least 18 years histologically or cytologically confirmed diagnosis. Web this template is intended to be used for clinical trials. Web clinical trial protocol doc. Cirm clinical protocol synopsis template study title provide full title of the study clinical phase specify clinical phase (1, 2a) study. The synopsis is your tool, your map to writing an excellent protocol,. Your protocol is the recipe for a successful trial. Clinical trial protocol pdf what is a clinical trial protocol clinical trial protocols are. Web students clinical trial protocol download our advanced review of the: Clinical trials are intended in their broadest sense and means any study design that involves an. Web download protocol synopsis template (dutch) patient facing documents can also be uploaded in this section of ctis. Clinical trial protocol version number: Web template protocol synopsis vs 2.0, dd march 2022 protocol synopsis (preferably in lay language, max. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared.Clinical Trial Protocol Template Australia Templates NjQyMDk
Clinical Trial Protocol Summary Template
Clinical Trial Protocol Template Ema
Clinical Study Protocol (CSP) Template Clinical Study Templates
Clinical Trial Protocol Template Eu Templates NjQyMjk Resume Examples
Clinical Trial Protocol Amendment Template
Ich Format for a Clinical Trial Protocol Clinical Trial Statistics
KHP CTU Protocol Template
Protocol Synopsis Template williamsonga.us
Clinical Trial Protocol
Web This Clinical Trial Protocol Template Is A Suggested Format For Phase 2 And 3 Clinical Trials Funded By The National Institutes Of Health (Nih) That Are Being Conducted Under A Food.
Center For Drug Evaluation And Research, Office Of Regulatory Policy.
Two Pages) Eu Trial Number And Full Trial Title Rationale Specify.
Web This Clinical Trial Protocol Template Is A Suggested Format For Phase 2 And 3 Clinical Trials Funded By The National Institutes Of Health (Nih) That Are Being Conducted Under A.
Related Post: