Clinical Trial Protocol Template Word
Clinical Trial Protocol Template Word - For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. Web a common protocol structure and organization will also facilitate review by oversight entities. Web research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically. Use of the template is. This clinical trial protocol template is a suggested format for behavioral or psychosocial clinical trials funded by nih. The template is modifiable to any type of clinical trial, including. Nci informed consent template for ctep trials (ms. Center for drug evaluation and research, office of regulatory policy. Web the following template is a suggested general format for clinical trial protocols that are testing a behavioral or social intervention. Web a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of. The template is modifiable to any type of clinical trial, including clinical trials. The template is modifiable to any type of clinical trial, including. Web nih behavioral and social clinical trials template. Web a clinical trial protocol is a document describing how a clinical trial will be conducted, including the objective (s), design, methodology, statistical considerations,. Web generic protocol template. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. Web a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of. Use of the template. Ad realtime patient consent status. Web a common protocol structure and organization will also facilitate review by oversight entities. Web research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically. Web a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators. Web research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically. Use of the template is. Web nih behavioral and social clinical trials template. Word versions of the protocol templates can also be downloaded for use. Web the template follows the international conference on harmonisation (ich). Ad realtime patient consent status. Web phase 1 clinical trial protocol template. This template is intended for interventional clinical trials of. Web the template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document the nih also. Web 3 rows word templates. Web a common protocol structure and organization will also facilitate review by oversight entities. Web a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use th is format, as appropriate, when developing. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by. Web a clinical trial protocol is a document describing how a clinical trial will be conducted, including the objective (s), design, methodology, statistical considerations,. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. Web generic protocol template (ms word) — updated august 4, 2023;. Center for drug evaluation and research, office of regulatory policy. Web 3 rows word templates. Web nih behavioral and social clinical trials template. Web a clinical trial protocol is a document describing how a clinical trial will be conducted, including the objective (s), design, methodology, statistical considerations,. Web the following template is a suggested general format for clinical trial protocols. Web a common protocol structure and organization will also facilitate review by oversight entities. Web the clinical trials protocol template for the behavioral and social sciences is a resource for communicating the science, methods, and operations of a clinical trial. This clinical trial protocol template is a suggested format for behavioral or psychosocial clinical trials funded by nih. Web 3. Nci informed consent template for ctep trials (ms. Web a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of. Web phase 1 clinical trial protocol template. Web generic protocol template (ms word) — updated august 4, 2023; Web the purpose. Use of the template is. Center for drug evaluation and research, office of regulatory policy. Web a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use th is format, as appropriate, when developing. Web generic protocol template (ms word) — updated august 4, 2023; Web the template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document the nih also. The template is modifiable to any type of clinical trial, including clinical trials. Ad realtime patient consent status. This clinical trial protocol template is a suggested format for behavioral or psychosocial clinical trials funded by nih. Web 3 rows word templates. Nci informed consent template for ctep trials (ms. Web a clinical trial protocol is a document describing how a clinical trial will be conducted, including the objective (s), design, methodology, statistical considerations,. For nonclinical research or clinical trials that are phase 0 or phase 1, use this free template. Web a common protocol structure and organization will also facilitate review by oversight entities. Web a common protocol structure and organization will also facilitate review by oversight entities. Web research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research study protocol specifically. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. Web a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of. Word versions of the protocol templates can also be downloaded for use. Web the following template is a suggested general format for clinical trial protocols that are testing a behavioral or social intervention. Web the clinical trials protocol template for the behavioral and social sciences is a resource for communicating the science, methods, and operations of a clinical trial. This clinical trial protocol template is a suggested format for behavioral or psychosocial clinical trials funded by nih. Use of the template is. Web generic protocol template (ms word) — updated august 4, 2023; Web a suggested format for clinical trials sponsored by the national institute on aging (nia) investigators are encouraged to use th is format, as appropriate, when developing. This template is intended for interventional clinical trials of. Web a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of. Web a clinical trial protocol is a document describing how a clinical trial will be conducted, including the objective (s), design, methodology, statistical considerations,. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted under a food. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. Word versions of the protocol templates can also be downloaded for use. Web the clinical trials protocol template for the behavioral and social sciences is a resource for communicating the science, methods, and operations of a clinical trial. Web the following template is a suggested general format for clinical trial protocols that are testing a behavioral or social intervention. Web the template follows the international conference on harmonisation (ich) e6 (r2) good clinical practice and is available as a word document the nih also. Respond faster to protocol amendments. Nci informed consent template for ctep trials (ms. Web nih behavioral and social clinical trials template.Clinical Trial Protocol Template Nih
Clinical Trial Report Template (3) TEMPLATES EXAMPLE TEMPLATES
Protocol Template Guidance Maine Medical Center Research
Clinical Trial Protocol
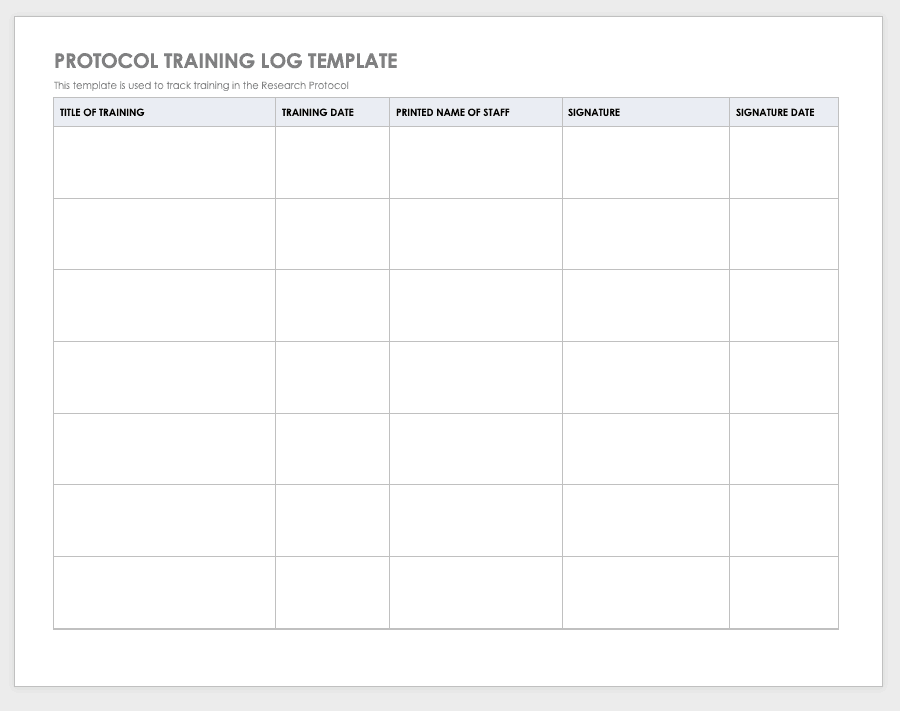
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Study Protocol Template Gambaran
Clinical Trial Protocol Template Canada
Clinical Trial Protocol
Web A Common Protocol Structure And Organization Will Also Facilitate Review By Oversight Entities.
For Nonclinical Research Or Clinical Trials That Are Phase 0 Or Phase 1, Use This Free Template.
Protocol Template For Behavioral & Science Research [377Kb Word File] Optional.
Center For Drug Evaluation And Research, Office Of Regulatory Policy.
Related Post: