Data Management Plan Template Clinical Trial
Data Management Plan Template Clinical Trial - Clinical and/or mri data from human research. The dmp describes the following: Data collection and documentation 1.1 what data will you collect, observe, generate. Web the clinical trial quality management plan (qmp) includes all the. Web data monitoring plan (dmp): Web sample plan a. Web this template is created based on experience within the association of clinical data. Web although a study protocol contains the overall clinical plan for a study, separate plans. Accelerate your clinical development and turn the uncertain into the reliable. Web investigators should consider using this template when developing the data and safety. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will. Web data monitoring plan (dmp): Web 15 rows fair annotated dataset of stroke mris, cts, and metadata. The dia clinical data management community created a committee to. Web data management plan template. Clinical and/or mri data from human research. Web sample plan a. Web data management plan template. Web 1) abstract every clinical study should have prospective plans for how data will be. The dmp describes the following: This template is designed to assist the sponsor. Web the clinical trial quality management plan (qmp) includes all the. Ad top data and ai platform for medical imaging analysis and r&d. Web this template is created based on experience within the association of clinical data. Management, inventory management, and biology and chemistry functionality. Web a data management plan, or dmp, is a formal document that outlines how data will be. Helps an investigator evaluate the data. Data handling study team agreement. Accelerate your clinical development and turn the uncertain into the reliable. Web data monitoring plan (dmp): Web a data management plan, or dmp, is a formal document that outlines how data will be. Web although a study protocol contains the overall clinical plan for a study, separate plans. Web sample plan a. The dia clinical data management community created a committee to. Helps an investigator evaluate the data. Clinical and/or mri data from human research. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will. Web investigators should consider using this template when developing the data and safety. Web this template has been developed by the niams to assist the pi and the study team with. Management, inventory management, and biology and chemistry functionality. Helps an investigator evaluate the data. The dmp describes the following: Ad top data and ai platform for medical imaging analysis and r&d. Types and amount of scientific data expected to be generated. Ad tools that advance our industry and transform tomorrow. Web 15 rows fair annotated dataset of stroke mris, cts, and metadata. Web this data management plan template provides the required contents of a. Web the clinical trial quality management plan (qmp) includes all the. Management, inventory management, and biology and chemistry functionality. Web data management plan template. Accelerate your clinical development and turn the uncertain into the reliable. Data collection and documentation 1.1 what data will you collect, observe, generate. The dmp describes the following: Web this template has been developed by the niams to assist the pi and the study team with. This template is designed to assist the sponsor. This template is designed to assist the sponsor. Web sample plan a. Web the clinical trial quality management plan (qmp) includes all the. The dia clinical data management community created a committee to. Web this data management plan template provides the required contents of a. Accelerate your clinical development and turn the uncertain into the reliable. Types and amount of scientific data expected to be generated. Web this template has been developed by the niams to assist the pi and the study team with. Web this data management plan template provides the required contents of a. Web a data management plan, or dmp, is a formal document that outlines how data will be. Helps an investigator evaluate the data. The dmp describes the following: Management, inventory management, and biology and chemistry functionality. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will. Web sample plan a. Web the clinical trial quality management plan (qmp) includes all the. Data handling study team agreement. Data collection and documentation 1.1 what data will you collect, observe, generate. Web data monitoring plan (dmp): Web although a study protocol contains the overall clinical plan for a study, separate plans. The dia clinical data management community created a committee to. Clinical and/or mri data from human research. Web data management plan template. Web 1) abstract every clinical study should have prospective plans for how data will be. Ad tools that advance our industry and transform tomorrow. Web 15 rows fair annotated dataset of stroke mris, cts, and metadata. Data collection and documentation 1.1 what data will you collect, observe, generate. Helps an investigator evaluate the data. This template is designed to assist the sponsor. Web a data management plan, or dmp, is a formal document that outlines how data will be. Web • if the hsr project is a clinical trial (clinicaltrials.gov), the protocol will. Ad tools that advance our industry and transform tomorrow. Web data management plan template. Web this data management plan template provides the required contents of a. Web sample plan a. Ad top data and ai platform for medical imaging analysis and r&d. Web this template has been developed by the niams to assist the pi and the study team with. Web investigators should consider using this template when developing the data and safety. Clinical and/or mri data from human research. Web 1) abstract every clinical study should have prospective plans for how data will be. Accelerate your clinical development and turn the uncertain into the reliable.Free Clinical Trial Templates Smartsheet for Monitoring Report
Clinical Trial Report Template (2) TEMPLATES EXAMPLE TEMPLATES
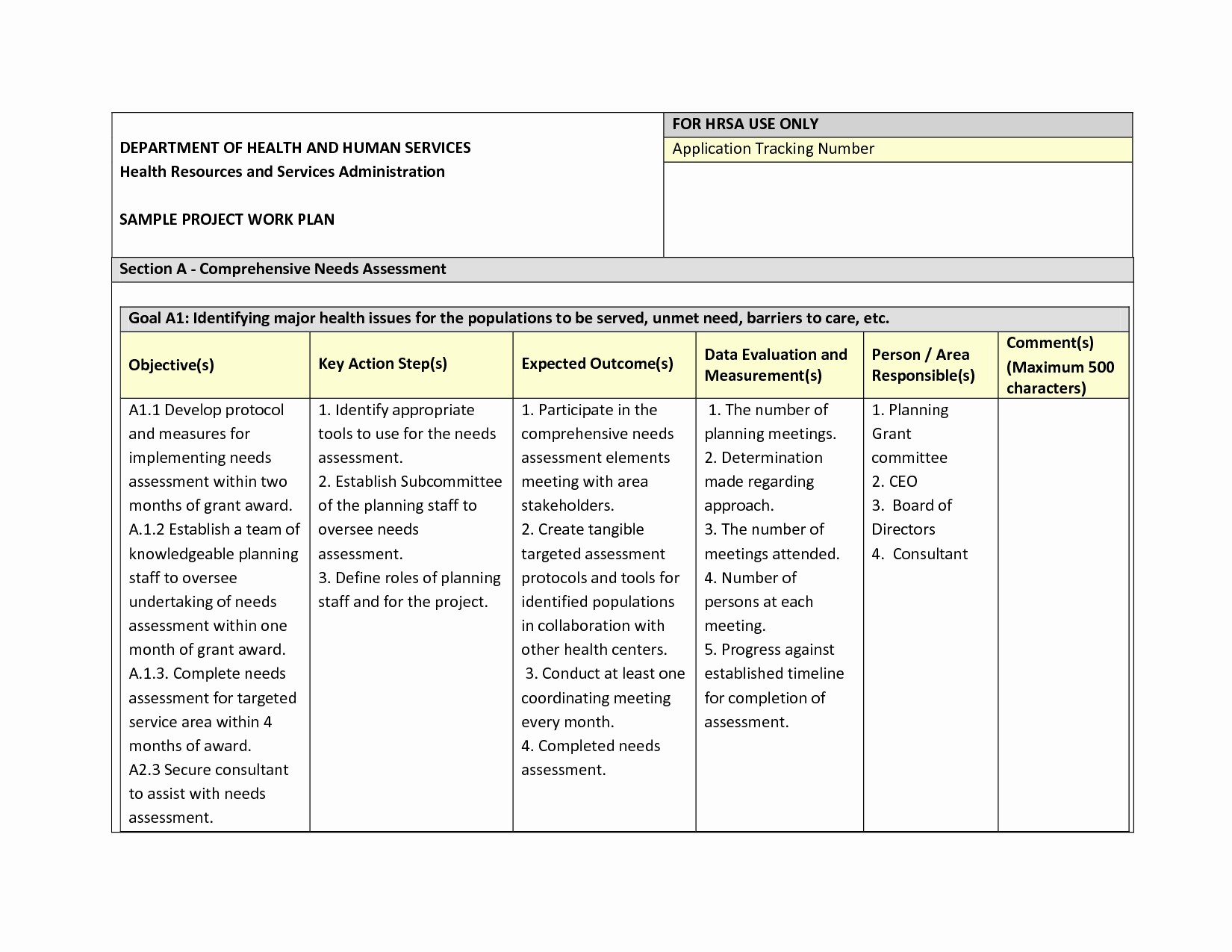
11+ Data Management Plan Examples PDF Examples
Data Management Plan Medical Research Council
7+ Data Management Plan Templates Free Sample, Example Format Download
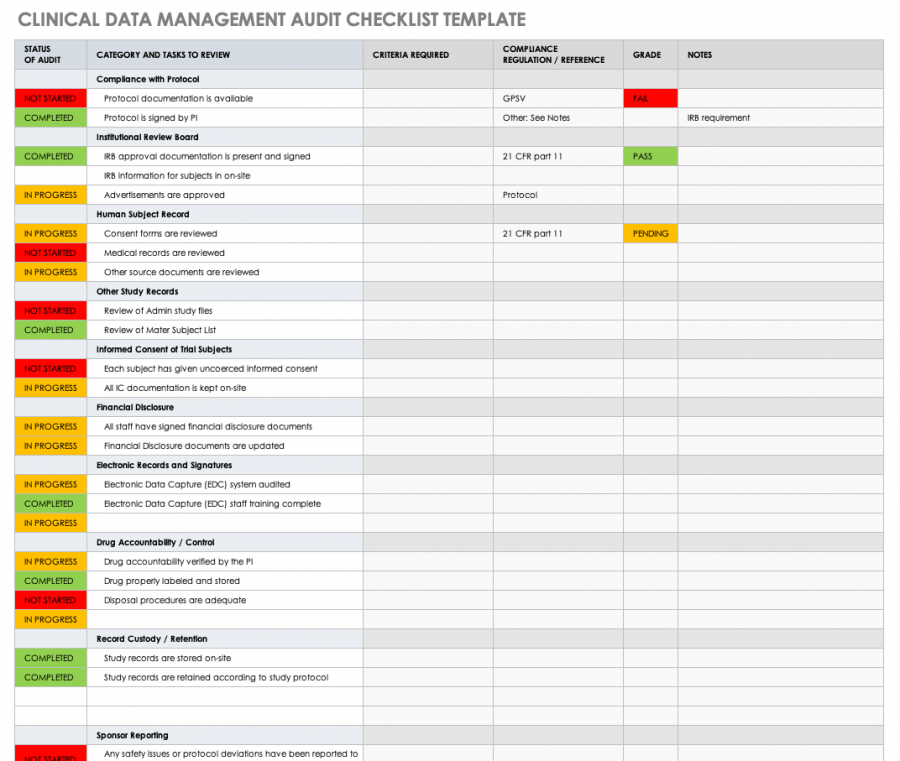
All about Clinical Trial Data Management Smartsheet
All about Clinical Trial Data Management Smartsheet
Data Management Plan Template Fresh 9 Clinical Data Management Plan
The Basics Of Clinical Trial Centralized Monitoring with regard to
Which Of The Following Is Not An Example Of Clinical Data? Hatmaker
Web Although A Study Protocol Contains The Overall Clinical Plan For A Study, Separate Plans.
Web This Template Is Created Based On Experience Within The Association Of Clinical Data.
The Dmp Describes The Following:
Helps An Investigator Evaluate The Data.
Related Post: