Drug Monograph Template
Drug Monograph Template - The aims of the drug monograph format will be to (1 ) ) measure the available proof of safety, tolerability, efficacy, cost,. The format supports the informed selection of drugs, tests, and devices by: Web download presentation development of drug monographs is a key responsibility for pharmacists at managed care organizations. Web 36 monograph drugs may be marketed without new drug applications approved under section 505 of 37 the fd&c act if they meet the requirements of section 505g of the. Web placement of new and existing drugs, tests, or devices or class of drugs, tests, or devices. Web the purpose of this monograph is to review the clinical data associated with the 26s proteasome inhibitor bortezomib approved in may 2003 for relapsed and refractory. Web • monographs provide an overview/evaluation of drugs, therapeutic classes and disease state therapies, to include efficacy, safety, cost information and. Web a monograph is a written document that reflects the quality attributes of medicines approved by the u.s. Web the purpose of this guidance document is to assist sponsors in developing product monographs with acceptable format and content. Web the purpose of va pbm services drug monographs is to provide a comprehensive drug review for making formulary decisions. Select one drug of your choice related to the topic being presented in class, and create a drug monograph for. The format supports the informed selection of drugs, tests, and devices by: Web drug monograph template. Web an otc drug monograph establishes conditions, such as active ingredients, uses (indications), doses, routes of administration, labeling, and testing, under which an otc.. Web drug monograph template. Web 36 monograph drugs may be marketed without new drug applications approved under section 505 of 37 the fd&c act if they meet the requirements of section 505g of the. Web placement of new and existing drugs, tests, or devices or class of drugs, tests, or devices. Web the purpose of this guidance document is to. Web drug evaluation monograph view table | | download (.pdf) summary: Food and drug administration (us fda). Clinical comparison (abstract at least two studies; Web simply stated, an otc monograph is a rule book for each therapeutic category establishing conditions, such as active ingredients, uses (indications), doses, route of. Web an otc drug monograph establishes conditions, such as active ingredients,. Web this scope includes drug products marketed under a final otc drug monograph, an approved nda or anda, and otc drug products for which there is no final otc drug. Web download presentation development of drug monographs is a key responsibility for pharmacists at managed care organizations. The format supports the informed selection of drugs, tests, and devices by: Select. Web how the drug, and similar drugs, fit into clinical guidelines. Web placement of new and existing drugs, tests, or devices or class of drugs, tests, or devices. Web the purpose of this guidance document is to assist sponsors in developing product monographs with acceptable format and content. Web drug evaluation monograph view table | | download (.pdf) summary: Artiblood. Web drug monograph template. Clinical comparison (abstract at least two studies; Web how the drug, and similar drugs, fit into clinical guidelines. Web • monographs provide an overview/evaluation of drugs, therapeutic classes and disease state therapies, to include efficacy, safety, cost information and. The aims of the drug monograph format will be to (1 ) ) measure the available proof. Web a monograph is a written document that reflects the quality attributes of medicines approved by the u.s. Web 36 monograph drugs may be marketed without new drug applications approved under section 505 of 37 the fd&c act if they meet the requirements of section 505g of the. Web • monographs provide an overview/evaluation of drugs, therapeutic classes and disease. Web the purpose of this monograph is to review the clinical data associated with the 26s proteasome inhibitor bortezomib approved in may 2003 for relapsed and refractory. Food and drug administration (us fda). Web this scope includes drug products marketed under a final otc drug monograph, an approved nda or anda, and otc drug products for which there is no. The format supports the informed selection of drugs, tests, and devices by: Web this scope includes drug products marketed under a final otc drug monograph, an approved nda or anda, and otc drug products for which there is no final otc drug. Web an otc drug monograph establishes conditions, such as active ingredients, uses (indications), doses, routes of administration, labeling,. Artiblood is a new perfluorocarbon that has many similarities to the only other product in its class, fakered. Web this scope includes drug products marketed under a final otc drug monograph, an approved nda or anda, and otc drug products for which there is no final otc drug. Web the purpose of this monograph is to review the clinical data. Web drug evaluation monograph view table | | download (.pdf) summary: Food and drug administration (us fda). The aims of the drug monograph format will be to (1 ) ) measure the available proof of safety, tolerability, efficacy, cost,. Web simply stated, an otc monograph is a rule book for each therapeutic category establishing conditions, such as active ingredients, uses (indications), doses, route of. Web a monograph is a written document that reflects the quality attributes of medicines approved by the u.s. These documents will be updated when new. Web an otc drug monograph establishes conditions, such as active ingredients, uses (indications), doses, routes of administration, labeling, and testing, under which an otc. Web placement of new and existing drugs, tests, or devices or class of drugs, tests, or devices. Atovaquone/proguanil restricted to cdc guidelines for treatment and prophylaxis of. Web the purpose of this monograph is to review the clinical data associated with the 26s proteasome inhibitor bortezomib approved in may 2003 for relapsed and refractory. Web the purpose of va pbm services drug monographs is to provide a comprehensive drug review for making formulary decisions. Clinical comparison (abstract at least two studies; Web • monographs provide an overview/evaluation of drugs, therapeutic classes and disease state therapies, to include efficacy, safety, cost information and. Web this scope includes drug products marketed under a final otc drug monograph, an approved nda or anda, and otc drug products for which there is no final otc drug. Web the purpose of this guidance document is to assist sponsors in developing product monographs with acceptable format and content. Web 36 monograph drugs may be marketed without new drug applications approved under section 505 of 37 the fd&c act if they meet the requirements of section 505g of the. Select one drug of your choice related to the topic being presented in class, and create a drug monograph for. Artiblood is a new perfluorocarbon that has many similarities to the only other product in its class, fakered. Web download presentation development of drug monographs is a key responsibility for pharmacists at managed care organizations. Web drug monograph template. Clinical comparison (abstract at least two studies; Web the purpose of this monograph is to review the clinical data associated with the 26s proteasome inhibitor bortezomib approved in may 2003 for relapsed and refractory. Web • monographs provide an overview/evaluation of drugs, therapeutic classes and disease state therapies, to include efficacy, safety, cost information and. Web drug evaluation monograph view table | | download (.pdf) summary: Food and drug administration (us fda). Web this scope includes drug products marketed under a final otc drug monograph, an approved nda or anda, and otc drug products for which there is no final otc drug. Web drug monograph template. Select one drug of your choice related to the topic being presented in class, and create a drug monograph for. Web a monograph is a written document that reflects the quality attributes of medicines approved by the u.s. Web the purpose of va pbm services drug monographs is to provide a comprehensive drug review for making formulary decisions. Web an otc drug monograph establishes conditions, such as active ingredients, uses (indications), doses, routes of administration, labeling, and testing, under which an otc. The format supports the informed selection of drugs, tests, and devices by: Web 36 monograph drugs may be marketed without new drug applications approved under section 505 of 37 the fd&c act if they meet the requirements of section 505g of the. Artiblood is a new perfluorocarbon that has many similarities to the only other product in its class, fakered. These documents will be updated when new. Web the purpose of this guidance document is to assist sponsors in developing product monographs with acceptable format and content.Drug Monograph
Linagliptin Drug Monograph Diabetes Management Insulin
Ahfs drug information monograph novabooks
Lorazepam (Drug Monograph)
Yervoy monograph for P&T
Customized communications in print media, customized medical journals
Drug Monograhs Template Drug Monograph DRUG MONOGRAPH OVERVIEW
Drug Monograph
Drug Monograph
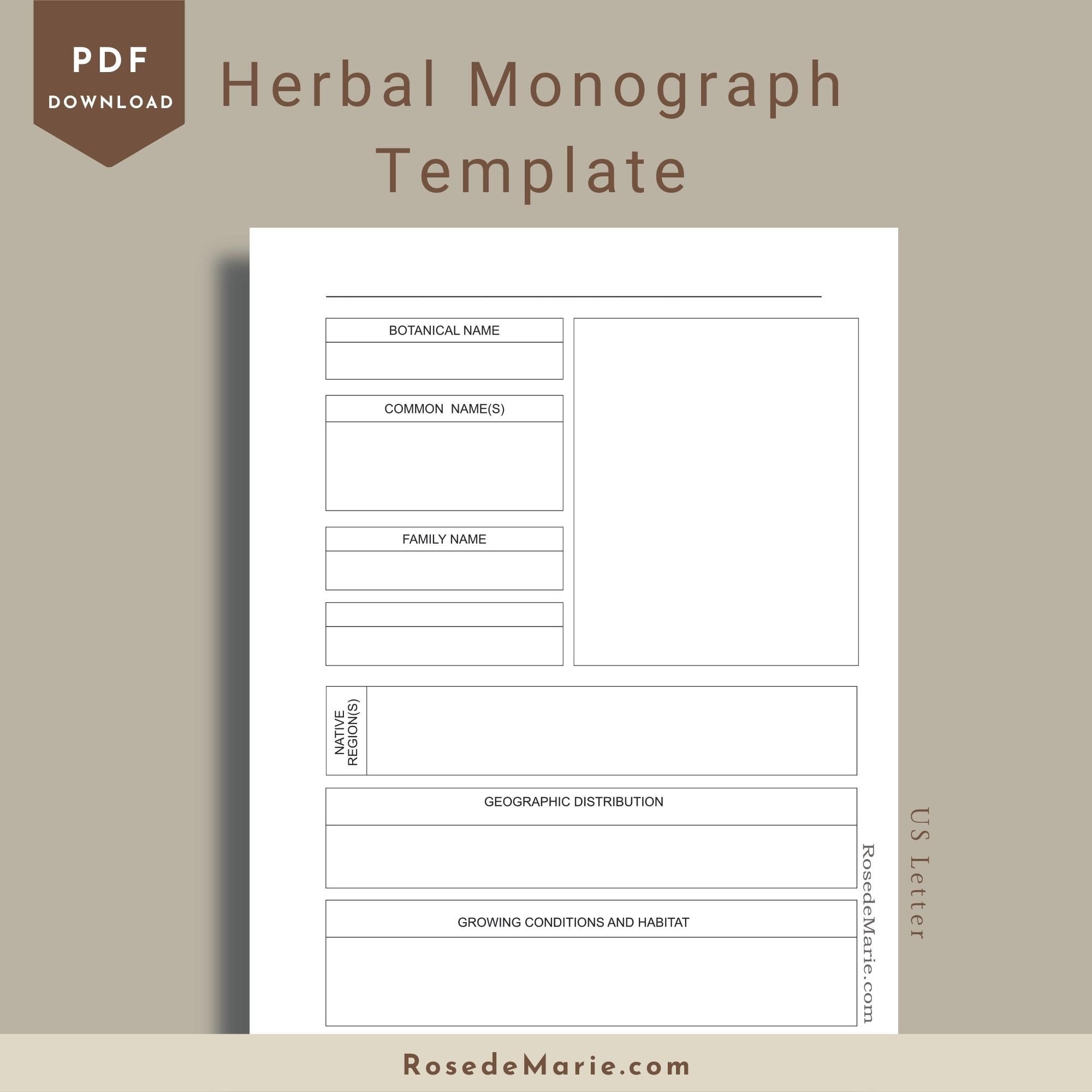
Herbal Monograph Template Materia Medica herbal Studies Etsy Australia
Web Simply Stated, An Otc Monograph Is A Rule Book For Each Therapeutic Category Establishing Conditions, Such As Active Ingredients, Uses (Indications), Doses, Route Of.
Web How The Drug, And Similar Drugs, Fit Into Clinical Guidelines.
The Aims Of The Drug Monograph Format Will Be To (1 ) ) Measure The Available Proof Of Safety, Tolerability, Efficacy, Cost,.
Atovaquone/Proguanil Restricted To Cdc Guidelines For Treatment And Prophylaxis Of.
Related Post: