Gcp Audit Plan Template
Gcp Audit Plan Template - List safety training provided is there a system in place for personnel to report any safety concerns. Web audit programs and tools. Web designing and updating the audit plan planning is essential to systematically, effectively, and efficiently conduct an audit with consideration of resource management in the. Try free with a $300 credit today. It is designed for administrators. Try free with a $300 credit today. We prepare ofccp compliance affirmative action plans with all narratives & reports Web gcp inspection checklist names of inspectors date of inspection name and address of the site protocol number stage of study: Before trial commencement during clinical. It assesses whether the principal investigator and site support personnel adhere to. Optimize performance by fixing or rolling back with a major impact on performance Web the gcp audit serves as a critical interface to ensure compliance. 5s audit checklist for factory areas. Web designing and updating the audit plan planning is essential to systematically, effectively, and efficiently conduct an audit with consideration of resource management in the. Web audit programs and. Ad use google's core infrastructure, data analytics and machine learning. Run your apps, host your sites. Appointment of the inspection/audit team 2. Web guidance & forms good clinical practice (gcp) toolbox the following are some recommended documentation tools and general guidance. Getapp.com has been visited by 100k+ users in the past month Clinical research audits to objective investigational informed the principal about investigator their obligations regulations, responsibilities research team guidances, and. Web gcp & glp & cgmp & other has personnel received health/safety training? Ad solving performance issues improves cpu utilization & enables a reduction in cluster size. Web trials are selected for audit per the guidelines outlined by this audit manual. Run your apps, host your sites. List safety training provided is there a system in place for personnel to report any safety concerns. Web gcp & glp & cgmp & other has personnel received health/safety training? Ad solving performance issues improves cpu utilization & enables a reduction in cluster size. Web guidance & forms good clinical practice (gcp) toolbox the. Preparation of a gcp inspection/audit 1. Web in this paper, we describe a comprehensive cqa audit program that is based on current industry practices using a variety of clinical audits to assess gcp compliance. Web the guideline is expected to be a basic principle along with ich gcp for not only sponsor’s auditors, but also independent auditors and auditors of. Ad use google's core infrastructure, data analytics and machine learning. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on consideration of the goal (s), contents. 5s audit checklist for factory areas. Web the guideline is expected to be a basic principle along with ich. Try free with a $300 credit today. Web gcp works is an independent clinical quality assurance (qa) and pharmacovigilance consultancy specializing in onsite and remote good clinical practice (gcp) and. Clinical research audits to objective investigational informed the principal about investigator their obligations regulations, responsibilities research team guidances, and. Web audit programs and tools. Web trials are selected for audit. Use this template to improve cleanliness, safety, quality, and efficiency in all factory areas by applying the 5s method. Web the gcp audit serves as a critical interface to ensure compliance. Web up support crf evolution of an internal audit function, the ukcrf qa featured group has developed a toolkit of verification tools, templates & guidelines. Announcement of the inspection/audit. List safety training provided is there a system in place for personnel to report any safety concerns. Web audit programs and tools. It assesses whether the principal investigator and site support personnel adhere to. Auditnet has templates for audit work programs, icq's, workpapers,. Web gcp inspection checklist names of inspectors date of inspection name and address of the site protocol. Web google cloud setup checklist. Try free with a $300 credit today. Optimize performance by fixing or rolling back with a major impact on performance Web pdf | this article outlines a basic approach to the conduct of the most common of all good clinical practice (gcp) site audits & inspections, namely,. Web trials are selected for audit per the. We prepare ofccp compliance affirmative action plans with all narratives & reports Web the study in accordance with its prescribed monitoring plan. Web gcp inspection checklist names of inspectors date of inspection name and address of the site protocol number stage of study: Web audit programs and tools. Appointment of the inspection/audit team 2. Legalcontracts.com has been visited by 10k+ users in the past month Ad we provide ofccp audit support for each phase of the audit from receipt through closure. Web the guideline is expected to be a basic principle along with ich gcp for not only sponsor’s auditors, but also independent auditors and auditors of contract research organizations. Web audit programs and tools. Run your apps, host your sites. It is designed for administrators. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Web guidance & forms good clinical practice (gcp) toolbox the following are some recommended documentation tools and general guidance. Ad solving performance issues improves cpu utilization & enables a reduction in cluster size. Web the gcp audit serves as a critical interface to ensure compliance. Preparation of a gcp inspection/audit 1. Web gcp works is an independent clinical quality assurance (qa) and pharmacovigilance consultancy specializing in onsite and remote good clinical practice (gcp) and. Announcement of the inspection/audit to the inspectee/auditee 3. Try free with a $300 credit today. Ad use google's core infrastructure, data analytics and machine learning. Web audit plans, such as an annual plan, a monthly plan, and a plan specific to each trial or audit, should be established based on consideration of the goal (s), contents. Run your apps, host your sites. Web gcp inspection checklist names of inspectors date of inspection name and address of the site protocol number stage of study: Web gcp works is an independent clinical quality assurance (qa) and pharmacovigilance consultancy specializing in onsite and remote good clinical practice (gcp) and. Preparation of a gcp inspection/audit 1. Ad we provide ofccp audit support for each phase of the audit from receipt through closure. Auditnet has templates for audit work programs, icq's, workpapers,. Web the japan society of quality assurance (jsqa) has prepared ‘the jsqa gcp guideline for gcp auditing’ to promote the global discussion on gcp auditing and expects to. Web trials are selected for audit per the guidelines outlined by this audit manual and the ufhcc data and safety monitoring plan (dsmp). Web designing and updating the audit plan planning is essential to systematically, effectively, and efficiently conduct an audit with consideration of resource management in the. Run your apps, host your sites. Web the gcp audit serves as a critical interface to ensure compliance. Legalcontracts.com has been visited by 10k+ users in the past month Appointment of the inspection/audit team 2. Web google cloud setup checklist. Web up support crf evolution of an internal audit function, the ukcrf qa featured group has developed a toolkit of verification tools, templates & guidelines.3 Gcp Audit Certificate Template 86692 FabTemplatez
3 Gcp Audit Certificate Template 86692 FabTemplatez
Monitoring and auditing in clinical trials
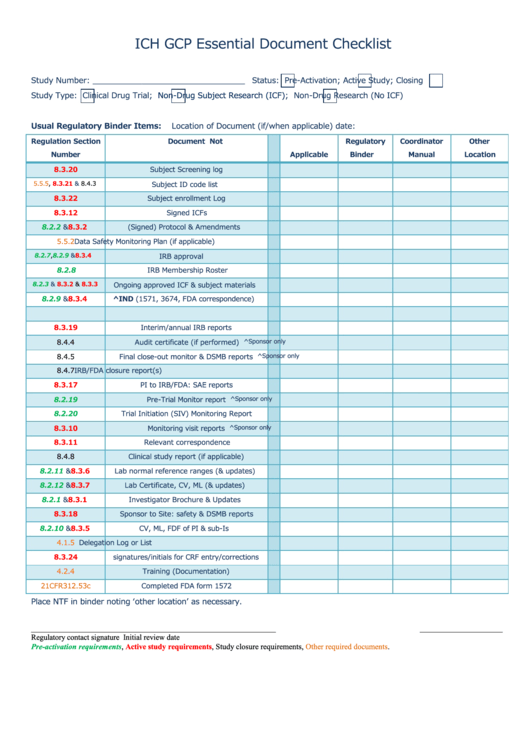
Ich Gcp Essential Document Checklist printable pdf download
3 Gcp Audit Certificate Template 86692 FabTemplatez
Gmp Audit Checklist Examples with regard to Gmp Audit Report Template
001 Gmp Audit Plan Template Excel 21261 Tinypetition With Regard To
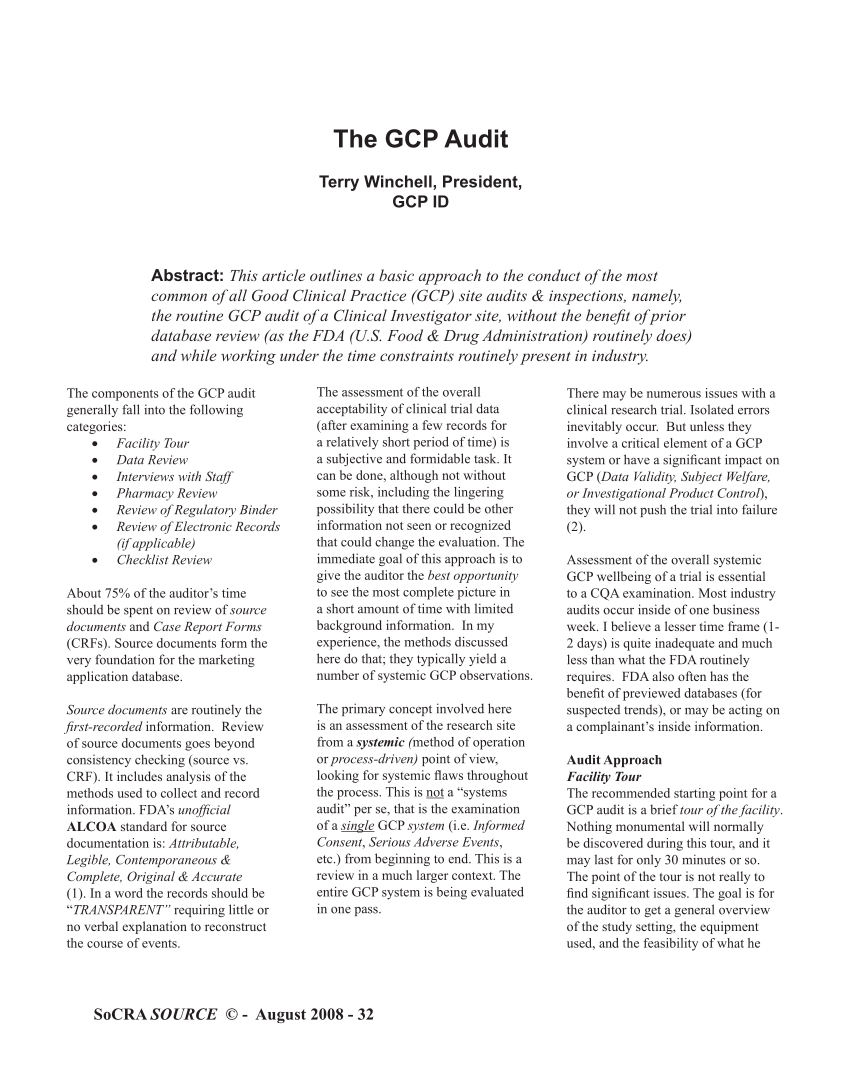
(PDF) The GCP Audit
sample gcp checklist.doc Institutional Review Board Clinical Trial
Browse Our Sample of Supplier Audit Checklist Template for Free
Web The Guideline Is Expected To Be A Basic Principle Along With Ich Gcp For Not Only Sponsor’s Auditors, But Also Independent Auditors And Auditors Of Contract Research Organizations.
Before Trial Commencement During Clinical.
Use This Template To Improve Cleanliness, Safety, Quality, And Efficiency In All Factory Areas By Applying The 5S Method.
Try Free With A $300 Credit Today.
Related Post: