Master Manufacturing Record Template
Master Manufacturing Record Template - Web we have added all the essential documents for agreements and contract to flowcharts related to manufacturing jobs in our template collection. What should a bmr contain? Web master batch records and batch production records. However, it’s so much more than just a place to. Stop wasting time & money. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. We have specified them by. ( a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Web master manufacturing record means, for each project, the template document proposed by paragon and approved by client that defines the final and complete manufacturing. I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). Web suitable for any size manufacturer, this template will enable you to track every detail from the moment you receive an order. Stop wasting time & money. Improve your manufacturing operations today w/ odoo. Web batch manufacturing records : Odoo mrp takes your manufacturing operations to the next level. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Master batch records, also known as master production records and master manufacturing records, are version. Odoo mrp takes your manufacturing operations to the next level. Not before creative the bmr, chemical and process manufacturers must make another document: This is. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of. Stop wasting time & money. Web the master production record is often referred to as the master batch record. What should a bmr contain? Master batch records, also known as master production records and master manufacturing records, are version. This is the documented and approved set of instructions used to describe how to manufacture a. Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even provide. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to. Web the master manufacturing record must include: However, it’s so much more than just a place to. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of. ( a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. (a) the name of. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). Web batch manufacturing records : Odoo mrp takes your manufacturing operations to the next. Stop wasting time & money. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. Web the master manufacturing record must include: Written instructions for a specific. Web i was just making a template for master manufacturing records template for the company i work for to help out production, and was hoping i could get some more. Master batch records, also known as master production records and master manufacturing records, are version. Web the master manufacturing record must include: Improve your manufacturing operations today w/ odoo. These. Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features to look for in a new system and even provide. Web master batch records and batch production records. Master batch records, also known as master production records and master manufacturing records, are version. (a) the name of the dietary. Web batch manufacturing records : To understand how to setup a master production record to understand the details of setting up the manufacturing. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. ( a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or. We have specified them by. Stop wasting time & money. Ad a modern solution to an old problem. Web the information on this page is current as of jan 17, 2023. These are required for each unique. Web manufacturing checklist templates and other work aids facilitate process planning, project management, production control, and quality inspection during the manufacturing. To understand how to setup a master production record to understand the details of setting up the manufacturing. (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Web the master manufacturing record must include: Web master your master batch records. What should a bmr contain? Web master batch records and batch production records. Written instructions for a specific manufacturing process. Web suitable for any size manufacturer, this template will enable you to track every detail from the moment you receive an order. Web any documents produced through the manufacturing process are then attached to the bmr as a record and proof of each stage. Web i was just making a template for master manufacturing records template for the company i work for to help out production, and was hoping i could get some more. Web master production records and batch production records have several professional aliases. This is the documented and approved set of instructions used to describe how to manufacture a. I am looking for templates of master production record (per 21 cfr part 211.186), and master batch record (per 21 cfr part 211.188). ( a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Stop wasting time & money. Web manufacturing checklist templates and other work aids facilitate process planning, project management, production control, and quality inspection during the manufacturing. This is the documented and approved set of instructions used to describe how to manufacture a. However, it’s so much more than just a place to. Web we have added all the essential documents for agreements and contract to flowcharts related to manufacturing jobs in our template collection. These are required for each unique. Ad a modern solution to an old problem. Web any documents produced through the manufacturing process are then attached to the bmr as a record and proof of each stage. Web the master manufacturing record must include: Web in accordance with 21 cfr part 211, pharmaceutical manufacturers are required to maintain their master batch records with certain details and information. Web the information on this page is current as of jan 17, 2023. Odoo mrp takes your manufacturing operations to the next level. To understand how to setup a master production record to understand the details of setting up the manufacturing. Not before creative the bmr, chemical and process manufacturers must make another document: (a) the name of the dietary supplement to be manufactured and the strength, concentration, weight, or measure of each dietary. Web master batch records and batch production records.PHARMACEUTICAL BATCH MANUFACTURING RECORD Sample Download M A N O X
Master Production Schedule Master Production Template
Great Master Production Schedule Excel Spreadsheet Job Application
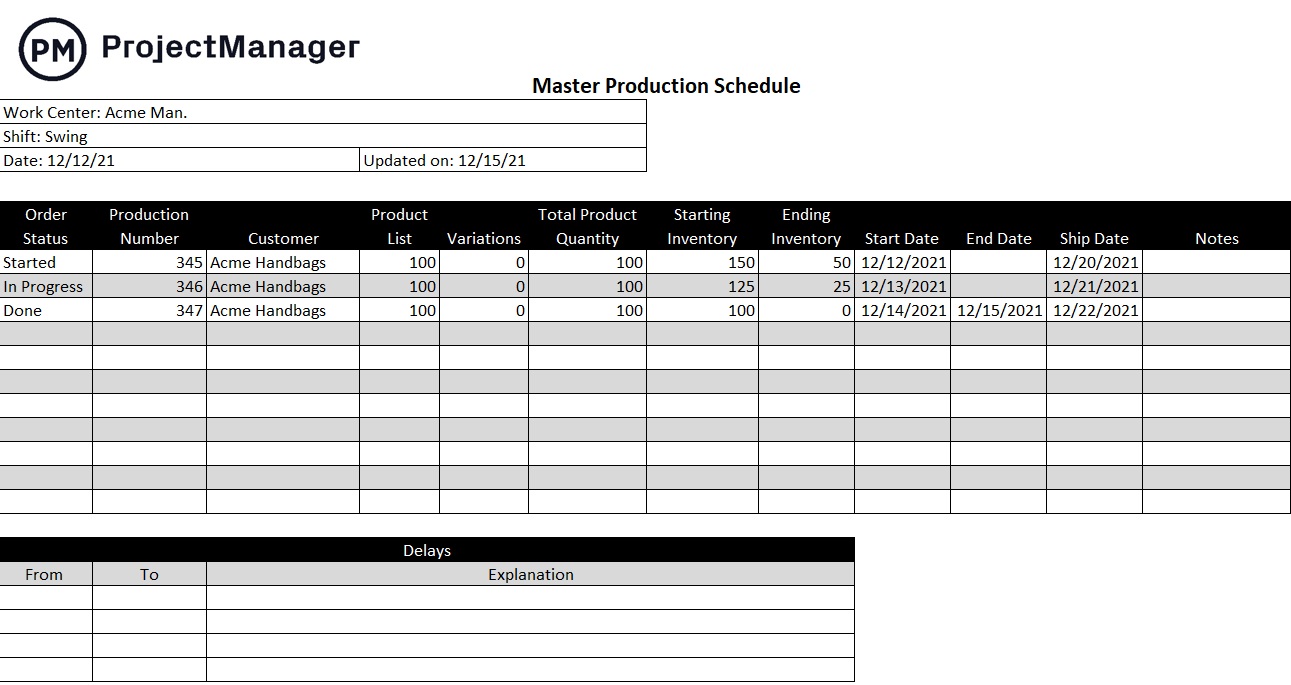
How to Create a Master Production Schedule (MPS) Project Manager News
Master Production Schedule Template Excel Luxury 29 Of Food
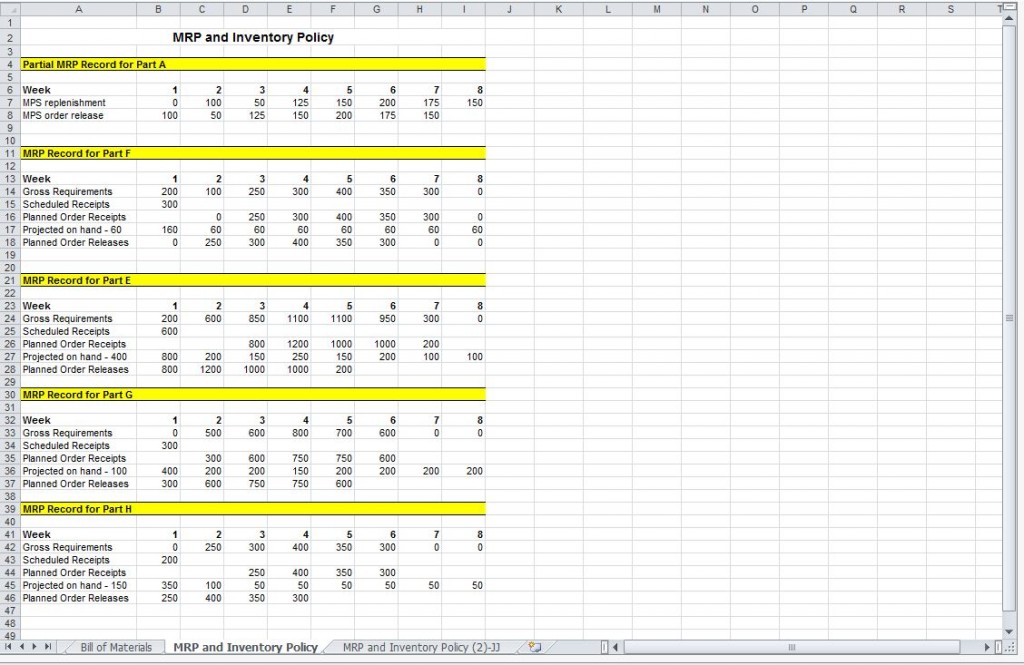
Mfr
Device Master Record Contents Template
Fotos De Doris Villallobos En Evaluación BF8
Device Master Record Procedure
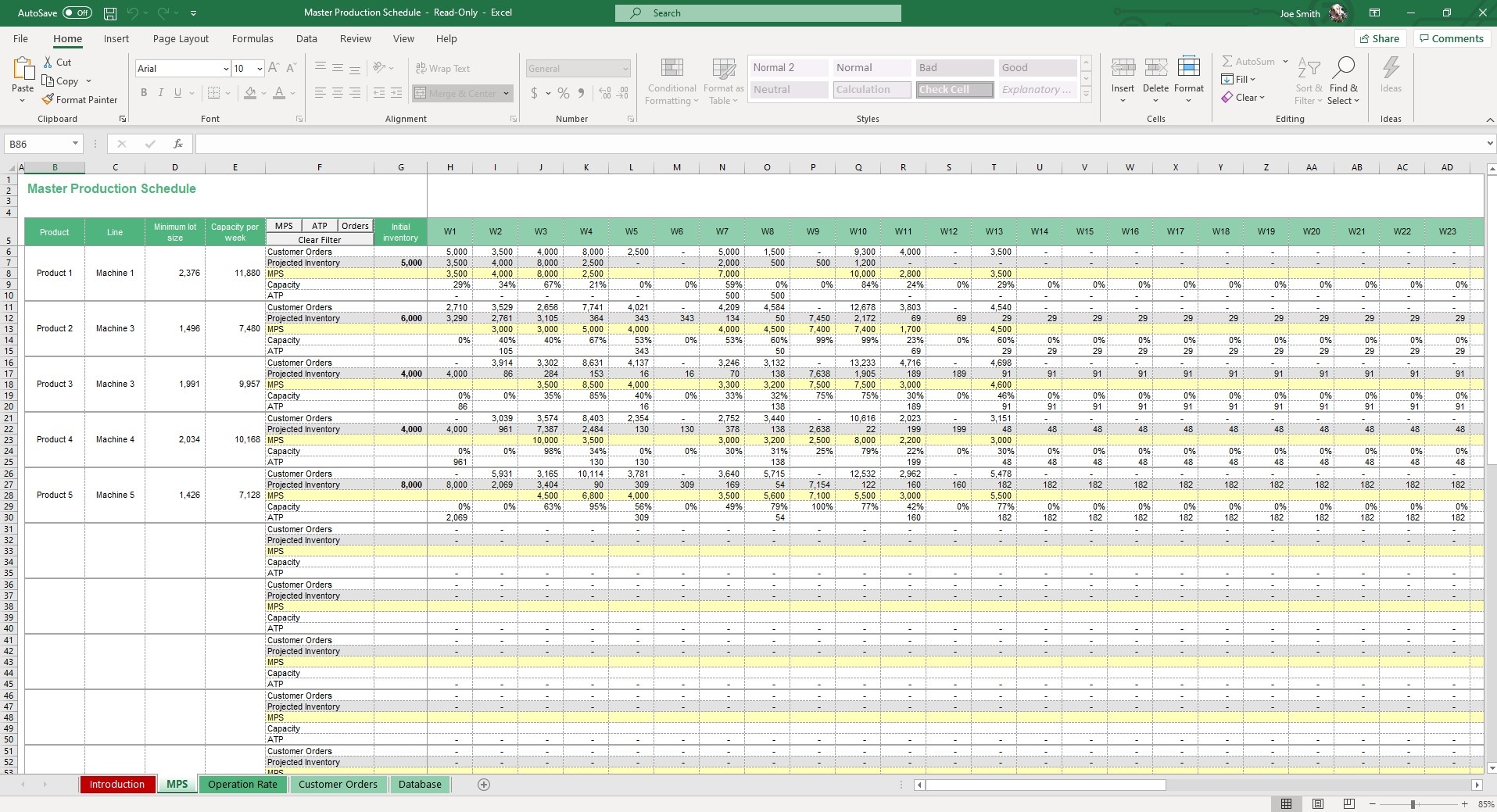
Master Production Schedule (MPS) Excel Template Simple Sheets
I Am Looking For Templates Of Master Production Record (Per 21 Cfr Part 211.186), And Master Batch Record (Per 21 Cfr Part 211.188).
Web In This Post, We’ll Show You How To Prepare A Batch Manufacturing Record, Walk You Through The Benefits And Features To Look For In A New System And Even Provide.
In The Manufacturing Industry, Master Production Records May Also Be Referred.
(A) The Name Of The Dietary Supplement To Be Manufactured And The Strength, Concentration, Weight, Or Measure Of.
Related Post: