Master Validation Plan Template
Master Validation Plan Template - With scribd, you can take your ebooks and audibooks anywhere, even offline. 5.2.7 for large projects involving many materials, a materials validation plan may be used. Click here to access our full library of downloadable content! You sack download a free sample of a validation master plan document in.pdf format. Current validation status for the systems within the project scope. Your pvp should contain the following elements: You can create a great protocol, using a template. Web what is a validation master planned? 9 pages the validation master plan is a high level plan that identifies all the computerized business systems at a site (or for an organizational unit). The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance criteria, and documenting the necessary program that ensures a continuing. Select to establish a validation master map previous the next fda audit? Web what is validation master plan (vmp): Appendices should contain all the relevant documentation referenced or stated in the. Web what is a validation master plan? Includes an overview of each process, the validation. Teach additional & download a free sample here Unlike a written plan for a specific validation project,. With scribd, you can take your ebooks and audibooks anywhere, even offline. You sack download a free sample of a validation master plan document in.pdf format. To see the complete list of the most popular. To see this complete list is and. Identify what needs to be validated. Web up to 30% cash back the scope of this validation master plan is limited to the following: You can download a free sample of a validation master plan template in.pdf format. Web what is a validation master planned? Web validation master plan examples. Web what is validation master plan (vmp): The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance criteria, and documenting the necessary program that ensures a continuing. Learn more & download a free sample here Identify what needs to be validated. Define device and the validation approach. Web the validation master plan (vmp) is critical in achieving this goal by documenting compliance requirements and explaining necessary validation activities across a manufacturing operation. Want more free medical device resources? This document comprises individual recommendations on four topics relating to equipment qualification and process. • the scope and intended use of the facility. Your pvp should contain the following elements: Web what is a validation master planned? Select to establish a validation master map previous the next fda audit? Web what is a validation master plan? • the scope and intended use of the facility • a. Current validation status for the systems within the project scope. Includes an overview of each process, the validation. Web validation master plan examples. Outlines the scope of the validation project and the strategy for validating the software’s installation and use. Your pvp should contain the following elements: Web up to 30% cash back the scope of this validation master plan is limited to the following: You sack download a free sample of a validation master plan document in.pdf format. How in create a validation master plan before the next fda audit? Web what is a validation master plan? Select to establish a validation master map previous the. Web what is a validation master plan? Web the validation master plan (vmp) is critical in achieving this goal by documenting compliance requirements and explaining necessary validation activities across a manufacturing operation. Outlines the scope of the validation project and the strategy for validating the software’s installation and use. Web a medical device validation master plan (vmp) diagrams the standards. Define device and the validation approach. 9 pages the validation master plan is a high level plan that identifies all the computerized business systems at a site (or for an organizational unit). Outlines the scope of the validation project and the strategy for validating the software’s installation and use. Web a medical device validation master plan (vmp) diagrams the standards. Systems, equipment, methods, facilities, etc., that are in the scope of the plan. Web the basic principles and application of qualification and validation are described in annex 15 to the pic/s and eu guide to gmp. For smaller projects, a materials validation plan is optional. Company new buildings b, c product a, b, c existing building a. Click here to access our full library of downloadable content! Appendices should contain all the relevant documentation referenced or stated in the. Web a medical device validation master plan (vmp) diagrams the standards associated with the capability of an office, characterizing the areas and systems to be approved, and gives a composed program for achieving and keeping up a certified. • the scope and intended use of the facility • a. Define device and the validation approach. To see the complete list of the most popular. Includes an overview of each process, the validation. Web what is a validation master plan? Web three (3) options to create a validation master plan. Try scribd free for 30 days. Learn more & download a free sample here The following topics will be discussed in this validation master plan: You can create a great protocol, using a template. Ad access millions of ebooks, audiobooks, podcasts, and more. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and acceptance criteria, and documenting the necessary program that ensures a continuing. You can download a free sample of a validation master plan template in.pdf format. Outlines the scope of the validation project and the strategy for validating the software’s installation and use. Your pvp should contain the following elements: Appendices should contain all the relevant documentation referenced or stated in the. To see the complete list of the most popular. How in create a validation master plan before the next fda audit? Ad access millions of ebooks, audiobooks, podcasts, and more. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Web what is validation master plan (vmp): Web what is a validation master plan? 5.2.7 for large projects involving many materials, a materials validation plan may be used. Company new buildings b, c product a, b, c existing building a. Learn more & download a free sample here Unlike a written plan for a specific validation project,. You can create a great protocol, using a template. 9 pages the validation master plan is a high level plan that identifies all the computerized business systems at a site (or for an organizational unit). Identify what needs to be validated.Data Validation Plan Template
How to create a Validation Master Plan in 5 steps. Templates & more
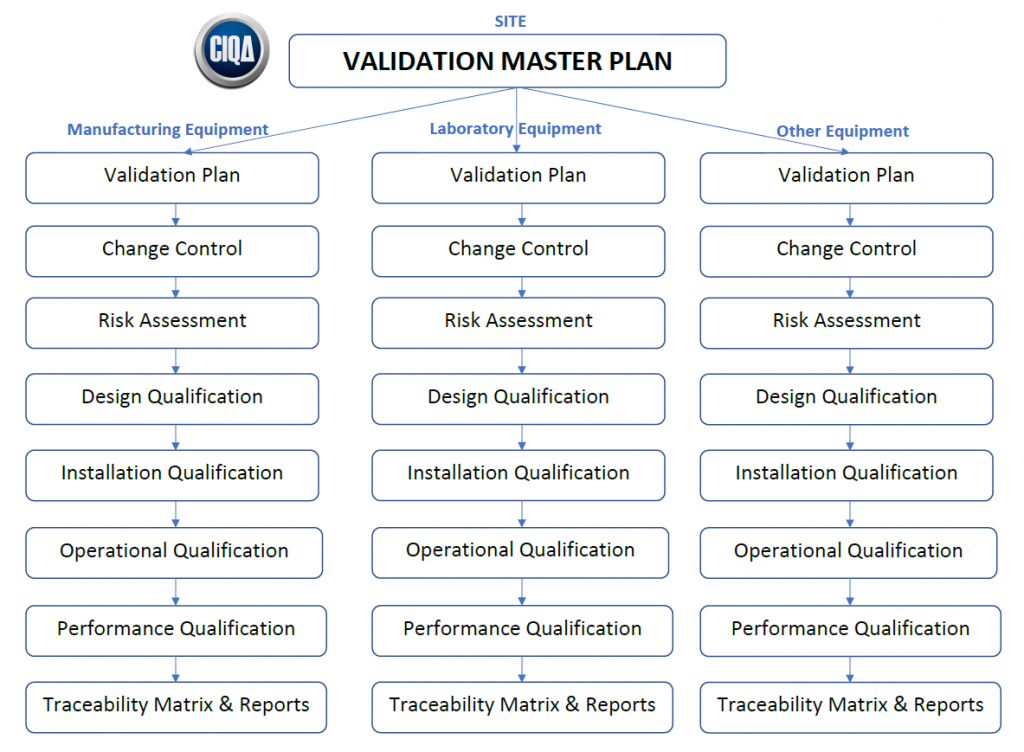
Master Validation Plan
Validation Master Plan Template Validation Center
Validation Master Plan
Validation Master Plan Template Pdf Best of Document Template
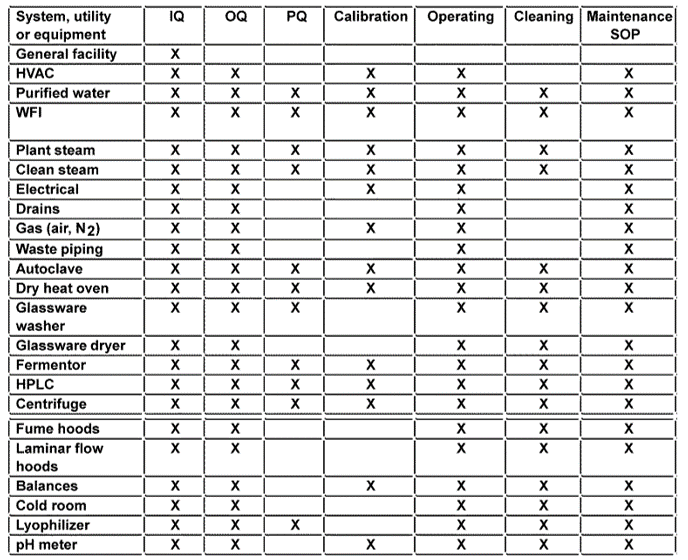
Example of a Validation Master Plan (VMP) Checklist Oriel STAT A
Template of a validation plan. Download Scientific Diagram
Validation Master Plan
Validation Master Plan
Web What Is A Validation Master Planned?
Teach Additional & Download A Free Sample Here
Select To Establish A Validation Master Map Previous The Next Fda Audit?
Define Device And The Validation Approach.
Related Post: