Validation Protocol Template
Validation Protocol Template - Web up to $3 cash back ii. Web it is critical to remember that the specifics of the topics covered in this section will be covered in the validation protocols. To determine that the equipment/system perform as intended by repeatedly running the system on its intended. It also serves as a. Web the validation plan and template provided in this document: It establishes a comprehensive plan to. The method validation plan template is one of the simplest and easiest templates that can help you define the scope and goals of a. Purpose of the method, parameters, equipment, procedures, criteria, timeline, and end users. The validation tasks are explained to the analyst(s) including: Web fda software validation template software validation for the chemical, manufacturing and cannabis industries what is software validation? That's why having a comprehensive validation protocol sop template is. Web use this process validation report template in the pharmaceutical industry to document everything properly. Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus avoiding. Use this equipment validation protocol template to report the. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services. Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. Web use. That's why having a comprehensive validation protocol sop template is. Web up to $3 cash back ii. It establishes a comprehensive plan to. It also serves as a. Validation protocol delete the sections which are not present in xxx system validation protocol, according to validations steps above. It is a example for the validation protocol. Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. Web it is critical to remember that the specifics of the topics covered in this section will be covered in the validation protocols. Web beginner ensuring the accuracy and. Web use this process validation report template in the pharmaceutical industry to document everything properly. To determine that the equipment/system perform as intended by repeatedly running the system on its intended. Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. Web this guidance outlines the general principles and approaches that fda. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. It also serves as a. Web use this process validation report template in the pharmaceutical industry to document everything properly. To determine that the equipment/system perform as intended by repeatedly running the system on its intended.. The validation tasks are explained to the analyst(s) including: Web up to $3 cash back ii. Validation protocol delete the sections which are not present in xxx system validation protocol, according to validations steps above. • guides the laboratory director in the establishment of method performance specifications considering the intended use. Purpose of the method, parameters, equipment, procedures, criteria, timeline,. Use this equipment validation protocol template to report the. It is a example for the validation protocol. Validation protocol delete the sections which are not present in xxx system validation protocol, according to validations steps above. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. •. Validation protocol delete the sections which are not present in xxx system validation protocol, according to validations steps above. Purpose of the method, parameters, equipment, procedures, criteria, timeline, and end users. Use this equipment validation protocol template to report the. It establishes a comprehensive plan to. The method validation plan template is one of the simplest and easiest templates that. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing facility. It is a example for the validation protocol. Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is transmitted by the ich assembly to the regulatory. The method validation plan. Purpose of the method, parameters, equipment, procedures, criteria, timeline, and end users. It is a example for the validation protocol. Web use this process validation report template in the pharmaceutical industry to document everything properly. The method validation plan template is one of the simplest and easiest templates that can help you define the scope and goals of a. Web the validation plan and template provided in this document: Use this equipment validation protocol template to report the. Web this equipment validation protocol template is designed to ensure that the equipment in question is safe, effective, and compliant with applicable regulations. The validation tasks are explained to the analyst(s) including: Web it is critical to remember that the specifics of the topics covered in this section will be covered in the validation protocols. Web beginner ensuring the accuracy and reliability of your processes is essential for any organization. Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services. Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. It establishes a comprehensive plan to. Web process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing facility. Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is transmitted by the ich assembly to the regulatory. Web fda software validation template software validation for the chemical, manufacturing and cannabis industries what is software validation? • guides the laboratory director in the establishment of method performance specifications considering the intended use. Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus avoiding 483s and warning letters. Web up to $3 cash back ii. Web it is critical to remember that the specifics of the topics covered in this section will be covered in the validation protocols. Web this guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal. Web fda software validation template software validation for the chemical, manufacturing and cannabis industries what is software validation? Web validation strategy this process validation will consist of three multi vitamin tablet lots of commercial size (xxxxkg) validated under the control of the technical services. Web up to $3 cash back ii. Web the validation, verification, and testing plan provides guidance for management and technical efforts throughout the test period. That's why having a comprehensive validation protocol sop template is. Web this protocol enables you to verify that your developed spreadsheet application is gmp compliant, thus avoiding 483s and warning letters. It is a example for the validation protocol. Web use this process validation report template in the pharmaceutical industry to document everything properly. • guides the laboratory director in the establishment of method performance specifications considering the intended use. Web beginner ensuring the accuracy and reliability of your processes is essential for any organization. Web this template is used to complete the process validation protocol by reporting the verification of the equipment/system final design against the user,. You can now validate your application. Web at step 2 of the ich process, a consensus draft text or guideline, agreed by the appropriate ich expert working group, is transmitted by the ich assembly to the regulatory. To determine that the equipment/system perform as intended by repeatedly running the system on its intended.Process Validation Templates at
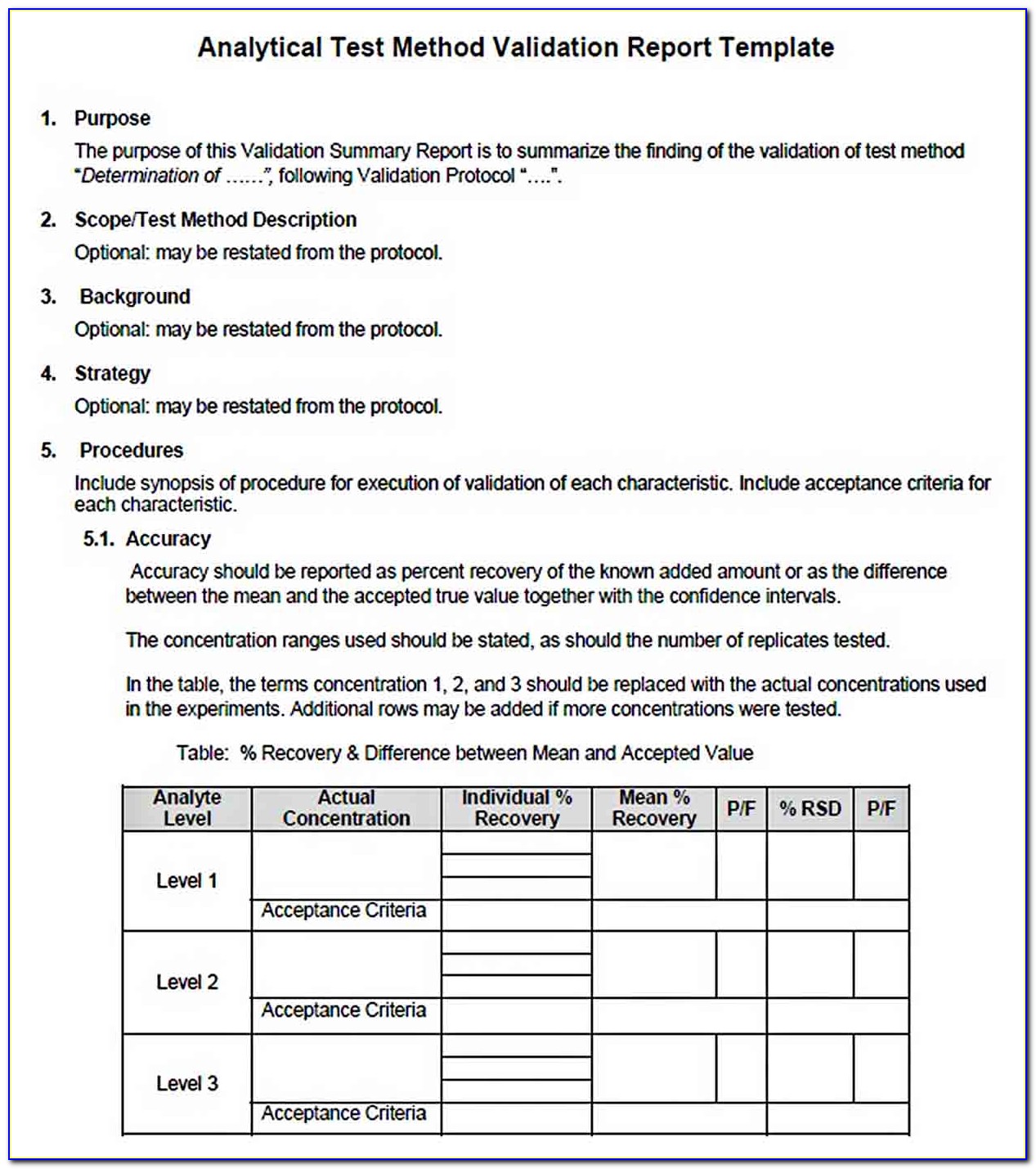
Analytical Method Validation Protocol Sample
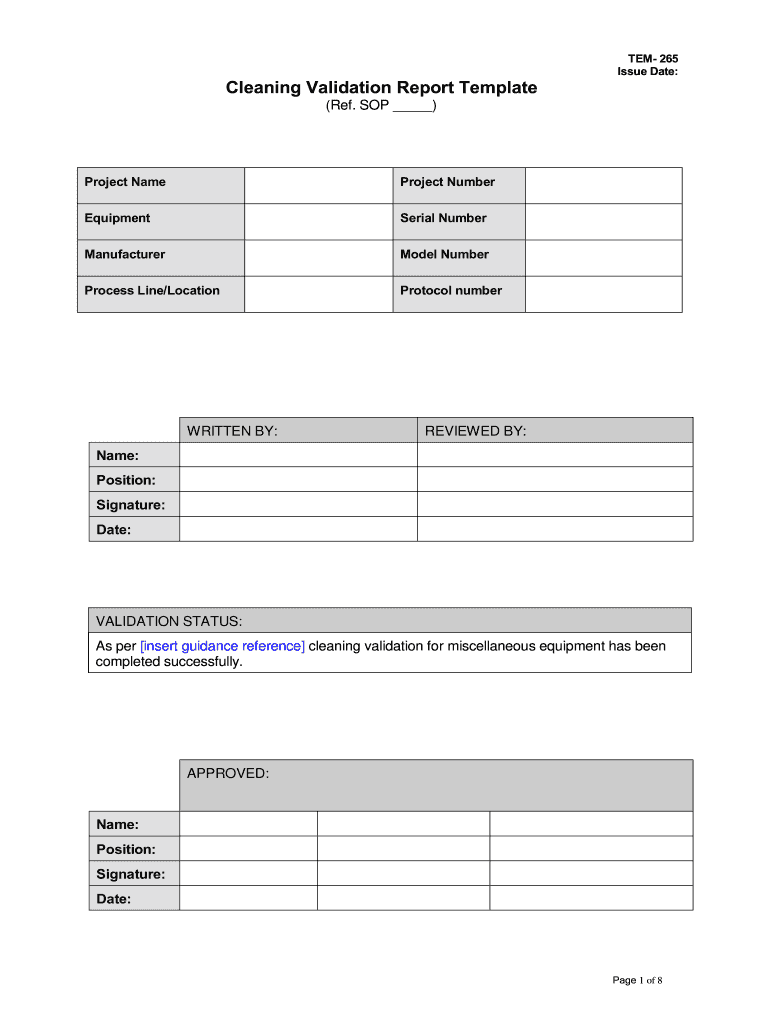
Cleaning Validation Report Template Fill Online, Printable, Fillable
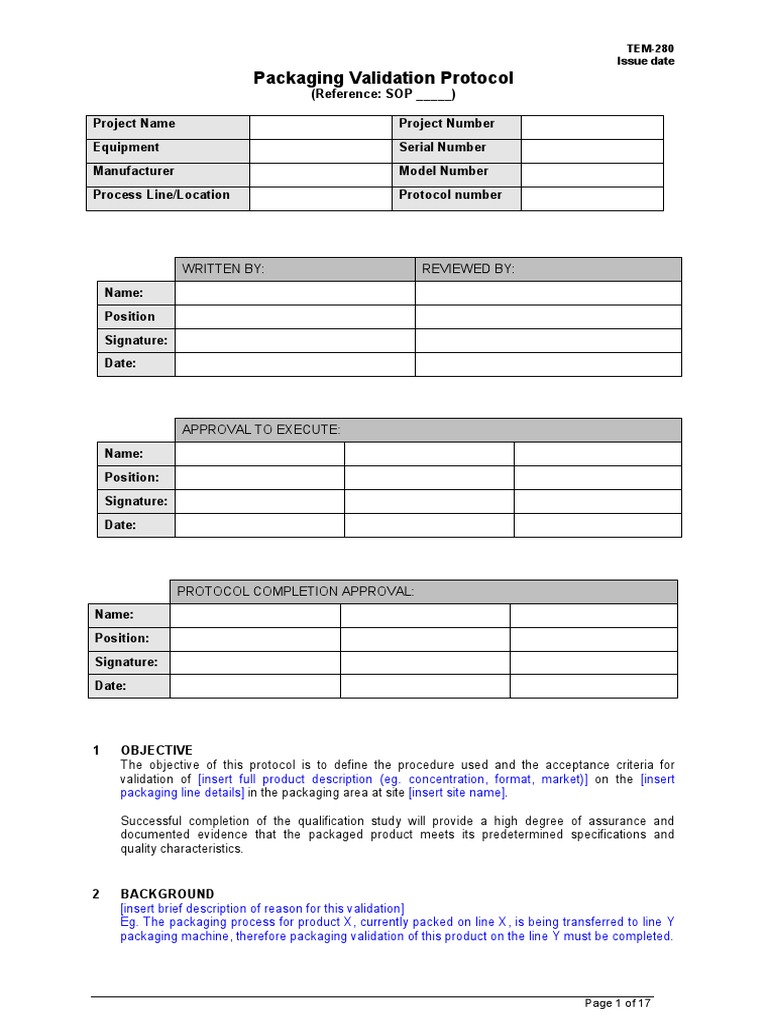
TEM280 Packaging Validation Protocol Template Sample Verification
Template of a validation plan. Download Scientific Diagram
Template for Process Validation Protocol Verification And Validation
Excel Spreadsheet Validation Protocol Template —
Sample Cleaning Validation Protocol
What's a Pharmaceutical Equipment Validation Protocol & Why is it Crucial?
Process Validation Protocol for Gliclazide Modified Release Tablets
Use This Equipment Validation Protocol Template To Report The.
Purpose Of The Method, Parameters, Equipment, Procedures, Criteria, Timeline, And End Users.
The Method Validation Plan Template Is One Of The Simplest And Easiest Templates That Can Help You Define The Scope And Goals Of A.
Web This Equipment Validation Protocol Template Is Designed To Ensure That The Equipment In Question Is Safe, Effective, And Compliant With Applicable Regulations.
Related Post: